A kind of preparation method of lactoforin
A technology of oxflufenapyr and acifluorfen, which is applied in the field of preparation of oxflufenapyr, can solve the problems of increasing the difficulty and capital expenditure of three-waste treatment, increasing the burden of three-waste treatment, complicated post-processing steps, and the like. The effect of eliminating the use of phase transfer catalysts, being easy to recycle and applying, and simplifying post-processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
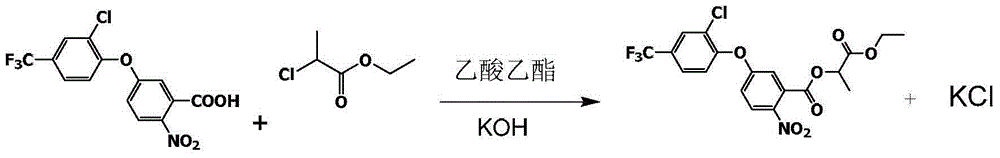
[0025] Into a 5000ml four-necked reaction bottle equipped with an azeotropic dehydration device, first put 2500ml of ethyl acetate as a solvent, and then add 1000g (2.76mol) of acifluorfen, 170g (3.02mol) of potassium hydroxide flakes, 2- Ethyl chloropropionate 440g (3.22mol). After the casting is completed, the temperature is raised, and the water generated by the reaction is removed by azeotropic dehydration. Keep warm at 80-85°C, and dehydrate the system while reacting. After the reaction of acifluorfen is complete, evaporate the ethyl acetate at a vacuum degree of -0.095MPa and a liquid temperature of ≦80°C, and finally pass through hot filtration (60 ~80°C), and the by-product potassium chloride was filtered off to obtain lactofen-methyl technical product, with an HPLC purity (retention time consistent with the standard product) of 88.3% and a yield of 98.2%.
[0026] The reaction formula is as follows
[0027]
Embodiment 2
[0029] Put 2500ml of solvent toluene into a 5000ml four-necked reaction bottle equipped with an azeotropic dehydration device, and then add 1000g (2.76mol) of acifluorfen, 170g (3.02mol) of potassium hydroxide, 2-chloropropane Ethyl acetate 440g (3.22mol). After the casting is completed, the temperature is raised, and the water generated by the reaction is removed by azeotropic dehydration. Keep warm at 90-100°C, and dehydrate the system while reacting. After the reaction of acifluorfen is complete, evaporate the toluene at a vacuum degree of -0.095MPa and a liquid temperature of ≦90°C, and finally pass through hot filtration (60-80 °C), and the formed by-product potassium chloride was filtered off to obtain lactofen-methyl technical product, with a HPLC purity (retention time consistent with the standard product) of 86.8% and a yield of 94.5%.
Embodiment 3
[0031] In a 5000ml four-necked reaction flask equipped with an azeotropic dehydration device, first put 2500ml of ethyl acetate as a solvent, and then add 1000g (2.76mol) of acifluorfen, 121g (3.02mol) of sodium hydroxide flakes, 2- Ethyl chloropropionate 440g (3.22mol). After the casting is completed, the temperature is raised, and the water generated by the reaction is removed by azeotropic dehydration. Keep warm at 80-85°C, dehydrate the system while reacting. After the reaction of acifluorfen is complete, evaporate the ethyl acetate at a vacuum degree of -0.095MPa and a liquid temperature of ≦80°C, and finally pass through hot filtration (60 ~80°C), and the formed sodium chloride was filtered off to obtain lactofen-methyl technical product, with a HPLC purity (retention time consistent with the standard product) of 85.3% and a yield of 93.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com