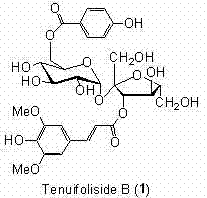
Synthetic method of Tenuifoliside B
A technology of acetoxyl and sucrose, applied in the field of chemical synthesis of natural product Tenuifolidise B, can solve the problem of no chemical synthesis of Tenuifolidise B, and achieve the effects of cheap and controllable cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
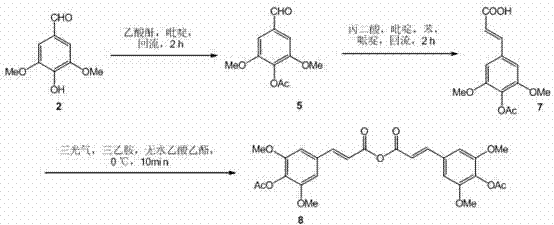
[0024] 4-Acetoxy-3,5-dimethoxycinnamic acid ( 7 ) preparation:
[0025] Syringaldehyde ( 2 ) 60.06 g (330 mmol) was dissolved in 360 mL of pyridine, 36.72 g (360 mmol) of acetic anhydride was added, refluxed for 2 hours, cooled to room temperature, and directly put into the next reaction, 500 mL of benzene, 52.00 g of malonic acid (500 mmol), piperidine 5 mL, install a reflux device with a water separator, oil bath, stir to dissolve, and reflux for 3 hours. After completion of the reaction, cool to room temperature, add 1000 mL of saturated sodium bicarbonate solution, stir, separate the water layer, acidify with 6 mol / L hydrochloric acid to a pH value of about 5, filter, rinse the filter cake with 500 ml of water, and dry to obtain White solid 4-acetoxy-3,5-dimethoxycinnamic acid ( 7 ) 56.18 g, yield 64%. Melting point, spectral data, and high-resolution mass spectrometry data of 4-acetoxy-3,5-dimethoxycinnamic acid: melting point 202-204 °C; = +0.2 (c = 1.01, MeOH); 1...
Embodiment 2
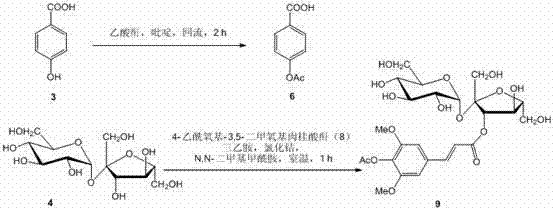
[0027] 4-acetoxybenzoic acid ( 6 ) preparation:
[0028] p-Hydroxybenzoic acid ( 3 ) 4.55 g (33 mmol) was dissolved in 30 mL of pyridine, 3.67 g (36 mmol) of acetic anhydride was added, and refluxed for 2 hours. After completion of the reaction, cool to room temperature, add 100 mL of saturated sodium bicarbonate solution, stir, separate the water layer, acidify with 6 mol / L hydrochloric acid to a pH value of about 2, filter, rinse the filter cake with 50 ml of water, and dry to obtain White solid 4-acetoxybenzoic acid ( 6 ) 5.50 g, the yield was 93%. Melting point, spectral data, and high-resolution mass spectrometry data of 4-acetoxybenzoic acid: melting point 191-193 °C; 1 H NMR (500 MHz, CD 3 OD): δ = 8.06 (m, J = 8.5, 2.5 Hz, 2 H), 7.21 (m, J = 8.5, 2.5 Hz, 2 H), 2.29 (s, 3 H) ppm; 13 C NMR (125 MHz, CD 3OD): δ = 170.7, 169.1, 156.1, 132.4 (2 C), 129.6, 123.0 (2 C), 21.0 ppm; HRMS (ESI): 181.0498 (calcd. for C 9 h 9 o 4 [M+H] + 181.0501).
Embodiment 3
[0030] 4-Acetoxy-3,5-dimethoxycinnamic anhydride ( 8 ):
[0031] 4-Acetoxy-3,5-dimethoxycinnamic acid ( 7 ) 31.92 g (120 mmol) was dissolved in 4000 mL of anhydrous ethyl acetate, 16.5 mL (120 mmol) of anhydrous triethylamine was added, cooled in an ice bath, and 5.94 g (20 mmol) of triphosgene was added at 0°C under stirring ), reacted for 10 minutes; reacted at room temperature for 20 minutes. Filter, wash the filter cake with anhydrous ethyl acetate (1000 mL), combine the filtrate and washing liquid, evaporate the solvent, and dry to obtain a white solid 4-acetoxy-3,5-dimethoxycinnamic anhydride ( 8 ), directly into the next reaction without purification, the yield is about 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com