Medicine preparation used for treating chronic cardiac failure and preparing method thereof
A pharmaceutical preparation and technology for chronic heart failure, applied in the field of traditional Chinese medicine granules for the treatment of chronic heart failure and its preparation, can solve the problems of difficult molding process operation, easy moisture absorption of extract powder, increased dosage, etc., and achieve simple process , improve patient compliance, the effect of less daily dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
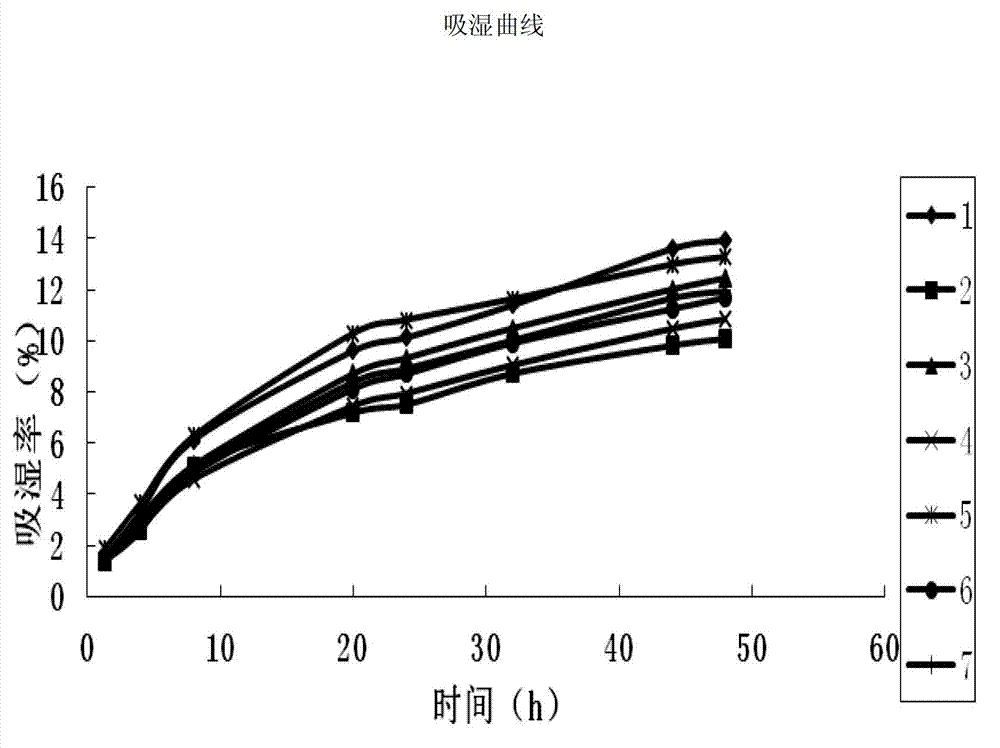
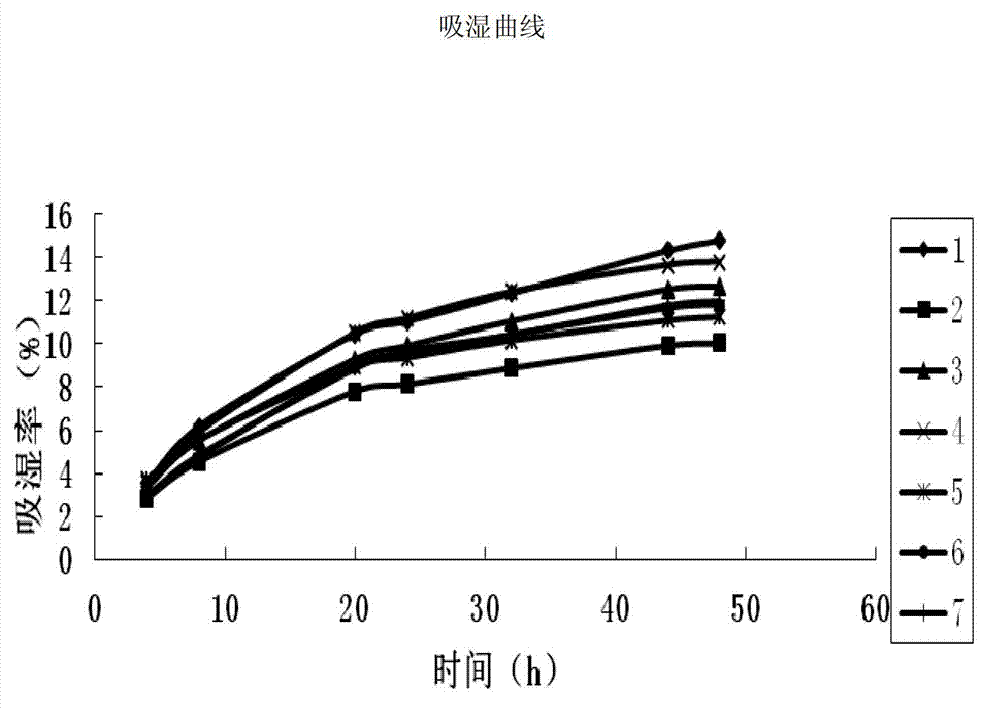
[0038] 1. Conditions of extract powder obtained by different drying methods
[0039] Traditional Chinese medicine extract powder has the characteristics of powder, and its particle size, fluidity, density, hygroscopicity, viscosity and other properties affect the formulation design, process selection and quality of finished products; among them, hygroscopicity, bulk density and fluidity It has a great influence on the compressibility of the powder, the effect of granulation, and the difference in particle loading.
[0040] In the present invention, the vacuum microwave drying and the vacuum drying methods are tested respectively when drying the extract, and compared with the spray drying method.
[0041] Take other raw materials in the formula except deer horn glue and add 10 times the amount of water, extract twice, 0.5 hours each time; combine the extracts, filter, concentrate the filtrate to a relative density of 1.02~1.04 (90°C), let cool, Centrifuge, take the supernatant...
Embodiment 2
[0070] Embodiment 2 Microwave drying prepares extract powder
[0071] Take 6Kg of Epimedium, 6Kg of Ligustrum lucidum, 4Kg of Psoralen, 3Kg of Tangerine Peel, and 3Kg of Cornus officinalis, add water and decoct three times, half an hour each time, add 10 times the amount of water each time, combine the decoction, filter, and concentrate the filtrate to The relative density is about 1.03~1.04 (90°C). Let it cool and centrifuge at high speed. Take the supernatant and concentrate it into an extract with a relative density of about 1.1 (90°C). Put it in a microwave dryer and microwave it at 55°C. Take it out after drying for 60 minutes, pulverize it to obtain extract powder, add deer horn gum powder, mix well to obtain medicinal powder, and dry granulate to obtain compound deer horn granules.
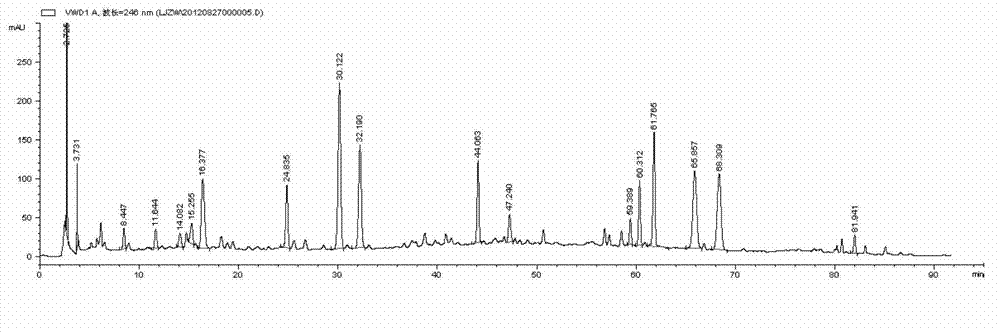
[0072] The HPLC method was used to determine the content of active ingredients and calculate the transfer rate, among which the transfer rate of icariin was 66%, and that of loganin was 60%...
Embodiment 3
[0073] Embodiment 3 Microwave drying prepares extract powder
[0074] Take 5Kg of Epimedium, 6Kg of Ligustrum lucidum, 3Kg of Psoralen, 2Kg of Tangerine Peel, 3Kg of Cornus officinalis, add 10 times the amount of water, decoct for one hour, filter; add 10 times the amount of water to the filter residue, decoct for one hour, filter After that, the secondary filtrate was combined and concentrated to a relative density of about 1.03 (90°C), centrifuged at 16000rps at a high speed, the supernatant was taken and then concentrated to an extract with a relative density of 1.12 (90°C), placed in a microwave dryer, at 50 Microwave drying at ℃ for about 80 minutes, take out and pulverize, add antler gum powder, mix well to obtain medicinal powder, dry granulate to obtain compound antler granules.
[0075] The HPLC method was used to determine the content of active ingredients and calculate the transfer rate, among which the transfer rate of icariin was 65%, and that of loganin was 64%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com