One-dimensional organic semiconductor nanowire with fluorescence and photoconduction dual response for organic amine gas as well as preparation method and application of nanowire
An organic semiconductor and photoconductive technology, applied in fluorescence/phosphorescence, chemical instruments and methods, organic chemistry, etc., can solve problems such as low carrier mobility, fluorescence quenching, and difficulties in organic semiconductor nanomaterials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
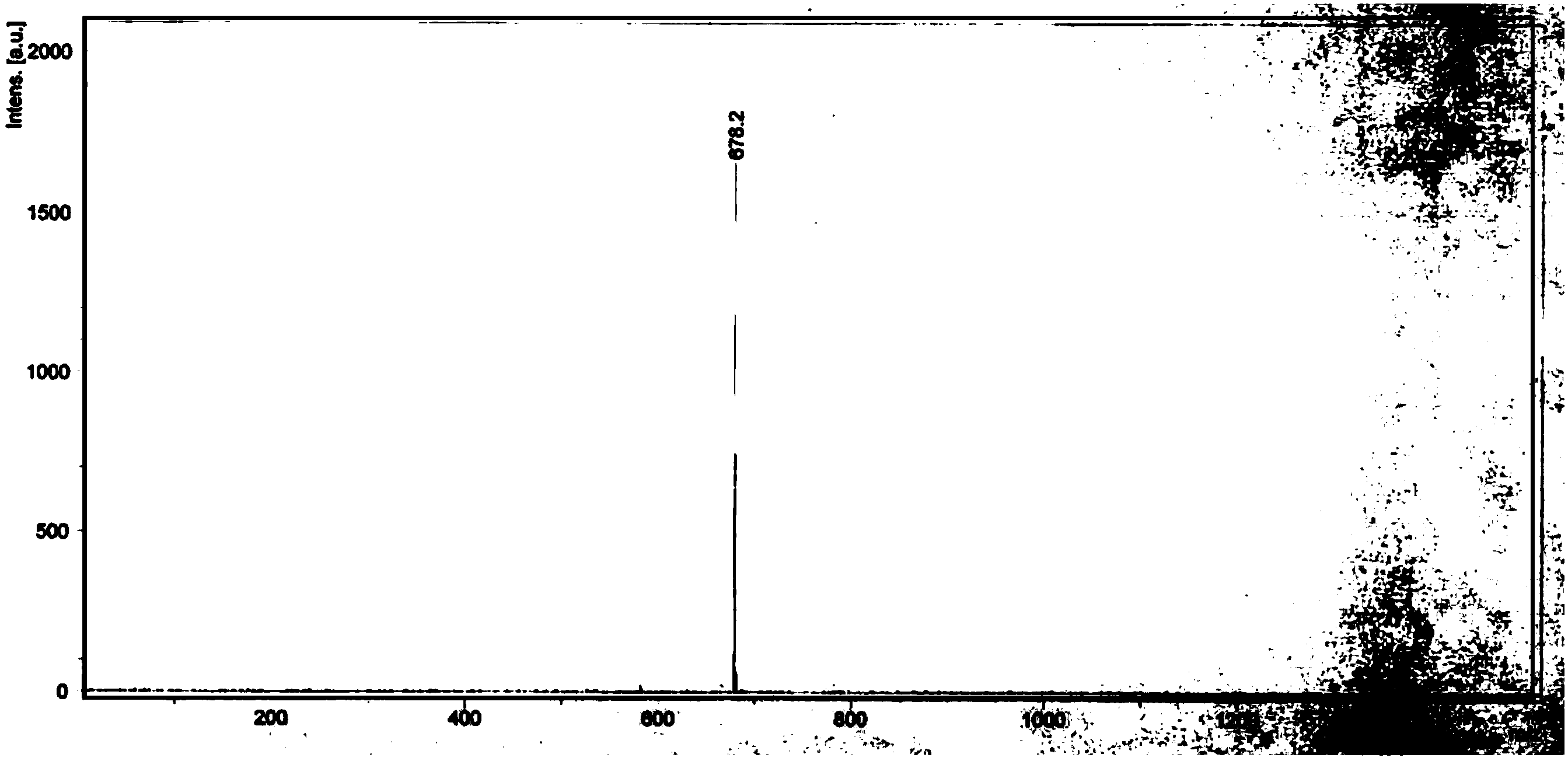
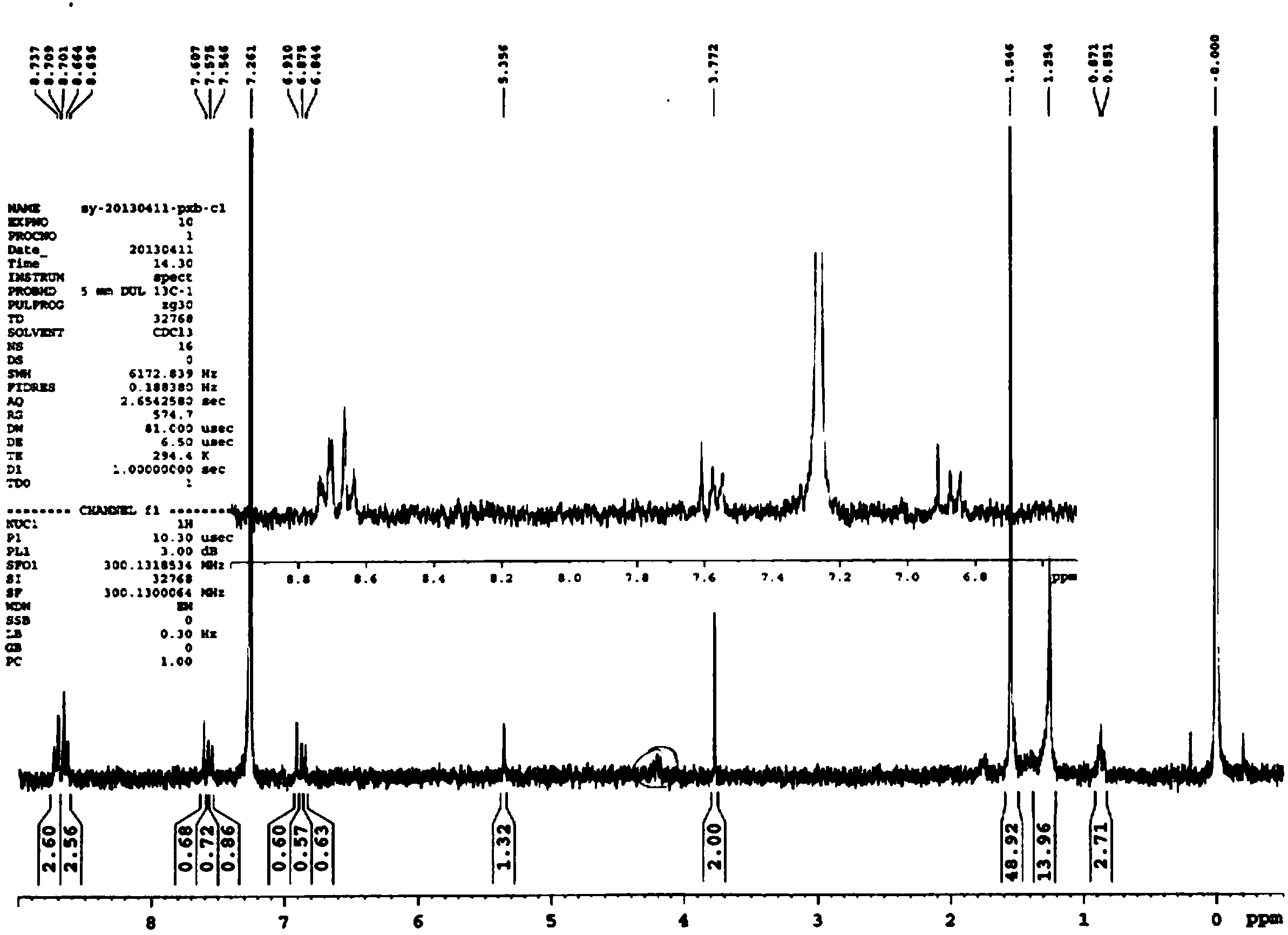
Image
Examples
Embodiment 1
[0050] (1) Mix 50-100 mg of perylene-3,4,9,10-tetracarboxylic dianhydride and 8 g of imidazole, heat to 130°C to dissolve, then inject slowly into dodecylamine for 3 hours of reaction The reaction solution was obtained, and then 10 ml of ethanol and 15 ml of concentrated hydrochloric acid (36% in mass concentration) were added to the reaction solution and stirred overnight; the product was taken out, rinsed with water until the pH was neutral, and then dried for use;
[0051] (2) Take 50 mg of the product obtained after drying in step (1), add 8 g of imidazole and 200 microliters of p-methoxybenzylamine to it, and react at a temperature of 130 ° C for 3 hours to obtain a reaction solution , then add 15 milliliters of concentrated hydrochloric acid (mass concentration is 36%) to the reaction solution, stir overnight, take out the product, and obtain the amphiphilic perylene imide derivative containing perylene anhydride as shown in the following structure; this containing peryle...
Embodiment 2
[0059] (1) Step (1) of the synthesis method is the same as step (1) of Example 1.
[0060] (2) Take 50 mg of the product obtained after drying in step (1), add 8 g of imidazole and 200 microliters of 3,5-dimethoxybenzylamine to it, and react at a temperature of 130 ° C for 3 hours Obtain the reaction liquid, then add 15 milliliters of concentrated hydrochloric acid (mass concentration: 36%) to the reaction liquid, stir overnight, take out the product, and obtain the amphiphilic perylene imide derivative containing perylene anhydride as shown in the following structure;
[0061]
[0062] (3) Dissolve the amphiphilic perylene imide derivative containing perylene anhydride obtained in step (2) in chloroform, then add ethanol (the volume ratio of chloroform to ethanol is 1:1), let it stand still, and prepare multiple The amphiphilic perylene imide derivatives of anhydrides are self-assembled through the π-π interaction between the perylene anhydrides to obtain dark blue one-dim...
Embodiment 3
[0066] The organic amine vapor in the air is detected by using the porous membrane of a plurality of red one-dimensional organic semiconductor nanowires self-assembled and woven to form a network structure prepared in Example 1; wherein: the concentration of n-hexylamine vapor is 50-70ppm; dibutylamine The concentration of steam is 10-20ppm; the concentration of triethylamine vapor is 330-360ppm, 3000-3500ppm respectively; the concentration of o-toluidine vapor is 2-8ppm; the concentration of aniline vapor is 3-8ppm.
[0067] A porous membrane composed of a plurality of red one-dimensional organic semiconductor nanowires self-assembled and woven to form a network structure is placed on a silicon dioxide sheet, so that the surface area of the porous membrane is very large, which is very suitable for gas molecules to flow on the porous membrane. Maximizes adsorption and facilitates diffusion and aggregation. The detected organic amine vapors are n-hexylamine with a concentrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com