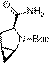Preparation method of saxagliptin intermediate
An intermediate and temperature control technology, which is applied in organic chemistry and other fields, can solve problems such as complex operation, high cost, and strict temperature control requirements, and achieve the effects of reasonable process conditions, increased final yield, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
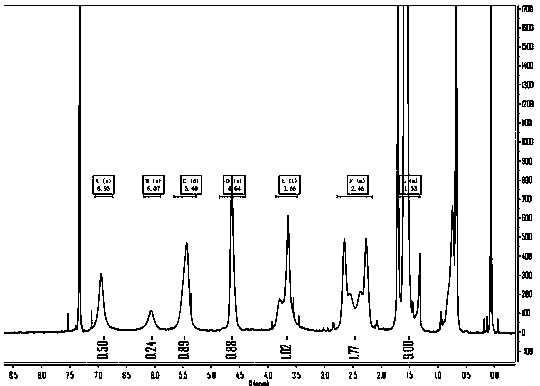
[0033] A preparation method of saxagliptin intermediate (1S, 3S, 5S)-tert-butyl-3-carbamoyl-2-azabicyclo[3.1.0]hexane-2-carboxylate, specifically Including the following steps:
[0034] (1), Preparation of Carbene Reagent A
[0035] Under nitrogen protection, 82g (1.25mol) of zinc powder, 18g (0.125mol) of cuprous bromide, 0.14g (0.6mmol) of iodine particles were successively added to a clean and dry four-necked flask, and 125mL of diethyl ether was added dropwise while stirring. Diiodomethane 0.1g (0.37mmol), heat up to a slight reflux state to initiate the reaction, after the reaction is initiated, the system will continue to exotherm and maintain a slight reflux for 10min, then control the temperature at 28-32°C, and add it at a rate of 1ml / min 151g (0.56mol) of diiodomethane, after the dropwise addition is completed, continue to stir at 28-32°C for 30min, and cool down to 0-5°C to obtain the carbene reagent A;
[0036] The amount of used catalyst iodine, cuprous bromide,...
Embodiment 2
[0047] A preparation method of saxagliptin intermediate (1S, 3S, 5S)-tert-butyl-3-carbamoyl-2-azabicyclo[3.1.0]hexane-2-carboxylate, specifically Include steps:
[0048] (1), Preparation of Carbene Reagent A
[0049] Under the protection of nitrogen, 82 g (1.25 mol) of zinc powder, 18 g (0.125 mol) of cuprous bromide, 0.14 g (0.6 mmol) of iodine particles were successively added to a clean and dry four-necked flask, and 125 mL of tetrahydrofuran was added. Add 0.1g (0.37mmol) of diiodomethane, and raise the temperature to a slight reflux state to initiate the reaction. After the reaction is initiated, the system will continue to exotherm and keep it for 10 minutes, then control the temperature at 28-32°C, and add it at a rate of 1ml / min. 151g (0.56mol) of diiodomethane, after the dropwise addition is completed, continue to stir at 28-32°C for 30min, and cool down to 0-5°C to obtain the carbene reagent A;
[0050] The amount of used catalyst iodine, cuprous bromide, Zn powder...
Embodiment 3
[0058] A preparation method of saxagliptin intermediate (1S, 3S, 5S)-tert-butyl-3-carbamoyl-2-azabicyclo[3.1.0]hexane-2-carboxylate, specifically Include steps:
[0059] (1), Preparation of Carbene Reagent A
[0060] Under nitrogen protection, 82 g (1.25 mol) of zinc powder, 18 g (0.125 mol) of cuprous bromide, 0.14 g (0.6 mmol) of iodine particles were successively added to a clean and dry four-necked flask, and 125 mL of diethyl ether was added, and stirred First add 0.1g (0.37mmol) of diiodomethane dropwise, and raise the temperature to a slight reflux state to initiate the reaction. After the reaction is initiated, the system will continue to exotherm and maintain a slight reflux for 10 minutes, then control the temperature at 28-32°C, and the drop rate is 1ml Add 151 g (0.56 mol) of diiodomethane per minute, after the dropwise addition, continue to stir at 55-60°C for 30 minutes, and cool down to 0-5°C to obtain carbene reagent A;
[0061] The amount of used catalyst io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com