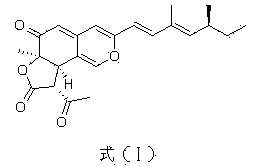
Application of compound Ochrephilone in preparing anti-tuberculosis medicine
A tuberculosis and drug technology, applied in the field of biology, can solve the problem of lack of effective anti-tuberculosis drugs and achieve the effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Fusion expression and purification of Mycobacterium tuberculosis serine / threonine protein kinase G protein.
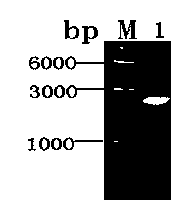
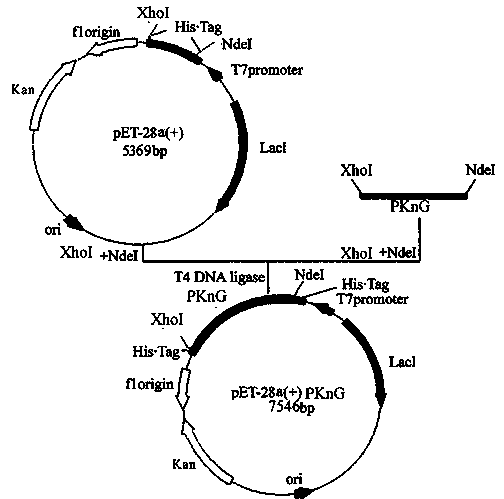
[0073] S1. The gene cloning of Mycobacterium tuberculosis serine / threonine protein kinase G was carried out according to the following steps: Genomic DNA of Mycobacterium tuberculosis H37Rv was extracted by phenol-chloroform extraction method, and according to the known Mycobacterium tuberculosis serine / threonine protein Kinase G gene sequence was designed and primers were synthesized, NdeI and XhoI restriction sites were added to the 5' ends of the upstream and downstream primers respectively, the pkng gene was cloned by PCR, and detected by 1.0% agarose gel electrophoresis, 2253 base pair long DNA fragment of Mycobacterium tuberculosis serine / threonine protein kinase G (see figure 1 ). Recover by electrophoresis (the kit for recovering DNA fragments from agarose gel is a product of Omega Company), purify the amplified gene fragments, dissolve them i...
Embodiment 2
[0081] Example 2 Analysis of the Inhibitory Enzyme Activity of Compound Ochrephilone on Mycobacterium tuberculosis Serine / Threonine Protein Kinase G
[0082] The ADP high-throughput kinase detection kit ADP-Glo® Kinase Assay Kit (product of Promega Company) was used to analyze the enzymatic activity of compounds inhibiting Mycobacterium tuberculosis serine / threonine protein kinase G, and the used Mycobacterium tuberculosis serine / threonine The amino acid protein kinase G is the recombinant Mycobacterium tuberculosis serine / threonine protein kinase G fusion protein obtained in Example 1. Add a certain amount of pure ATP, kinase reaction solution, recombinant mycobacterium tuberculosis serine / threonine protein kinase G in the 200ul PCR tube, incubate at room temperature for 20min, and prepare positive control, negative control and blank control wells at the same time, this embodiment The positive control used to inhibit the activity of Mycobacterium tuberculosis serine / threonine...
Embodiment 3
[0086] Example 3 Molecular docking software predictive analysis of the interaction between Ochrephilone and Mycobacterium tuberculosis serine / threonine protein kinase G
[0087] Using the SYBYL X molecular docking software, the protomol was used to represent the binding pocket of the protein. Use three kinds of probes to fill the surface properties of the probe binding pocket, and then carry out structural similarity matching, complementarity, interaction, H-bond acceptor and donor interaction between the small molecule ligand and the probe in the binding pocket during docking, Hydrophobic Interaction Domain Interaction, mimicking the interaction of Ochrephilone with Mycobacterium tuberculosis serine / threonine protein kinase G (see Figure 11 ), the score given by the software is 4.97, and generally a score greater than 4 indicates that there is an interaction, and the reliability is very high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com