B-containing nano crystalline Ru-based catalyst, preparation method and applications thereof
A catalyst and nanocrystalline technology, which is applied in the field of selective hydrogenation of benzene to cyclohexene containing B nanocrystalline Ru-based catalysts, can solve the problems of low cyclohexene selectivity, high material requirements, equipment corrosion, etc., and achieve Effect of high activity and cyclohexene selectivity, reduced requirements, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
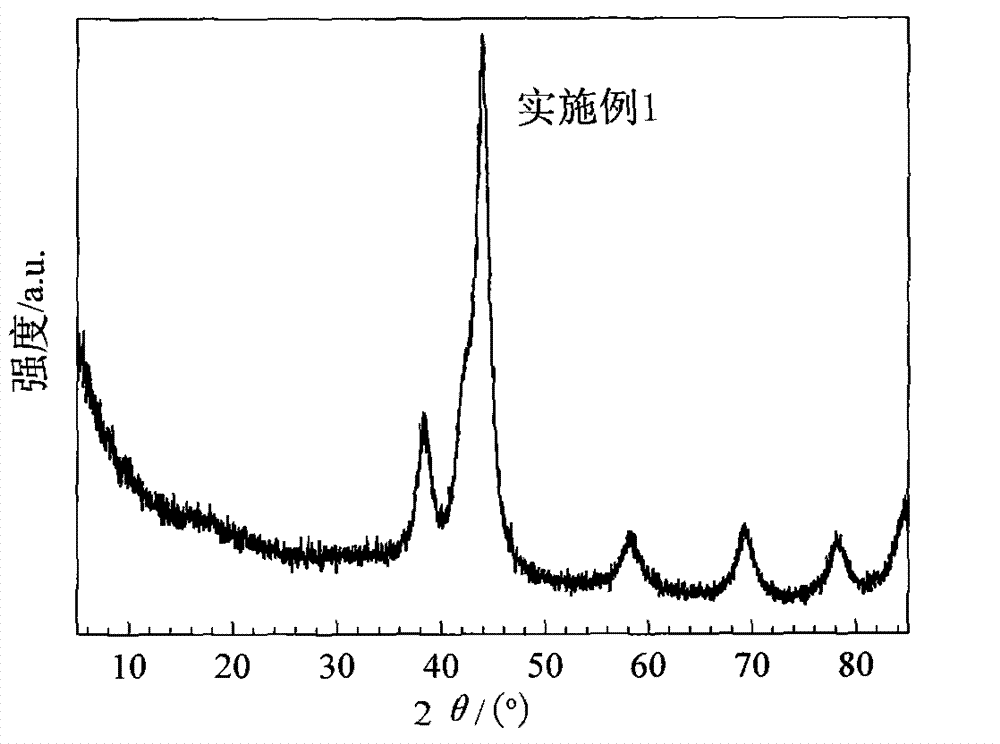
[0021] Mix 1.71g of ruthenium trichloride (containing 0.65g of metal Ru), 0.0242g of boric acid and 0.0024g of zinc oxide into 400ml of distilled water to prepare a slurry, and adjust its pH value to 6-7 with ammonia water. The above solution was poured into a 1L Hastelloy-lined reactor, and the temperature was raised to 100° C. for 5 hours, and the stirring rate was controlled at 200 r / min during the process. The slurry was cooled and taken out, and suction filtered until the pH of the filtrate=7. Mix the obtained solid and 400ml of distilled water into the reaction kettle, and reduce for 3h under the reducing conditions of hydrogen pressure of 2MPa, temperature controlled at 140°C and stirring speed of 1200r / min. The obtained solid is nano RuZnB crystalline alloy. The XRD spectrum of the catalyst is shown in figure 1 , the diffraction peaks at 38.3°, 43.8°, 58.2°, 69.4° and 78.1° in the figure all belong to the characteristic peaks of crystalline metal Ru. It can be seen ...
Embodiment 2
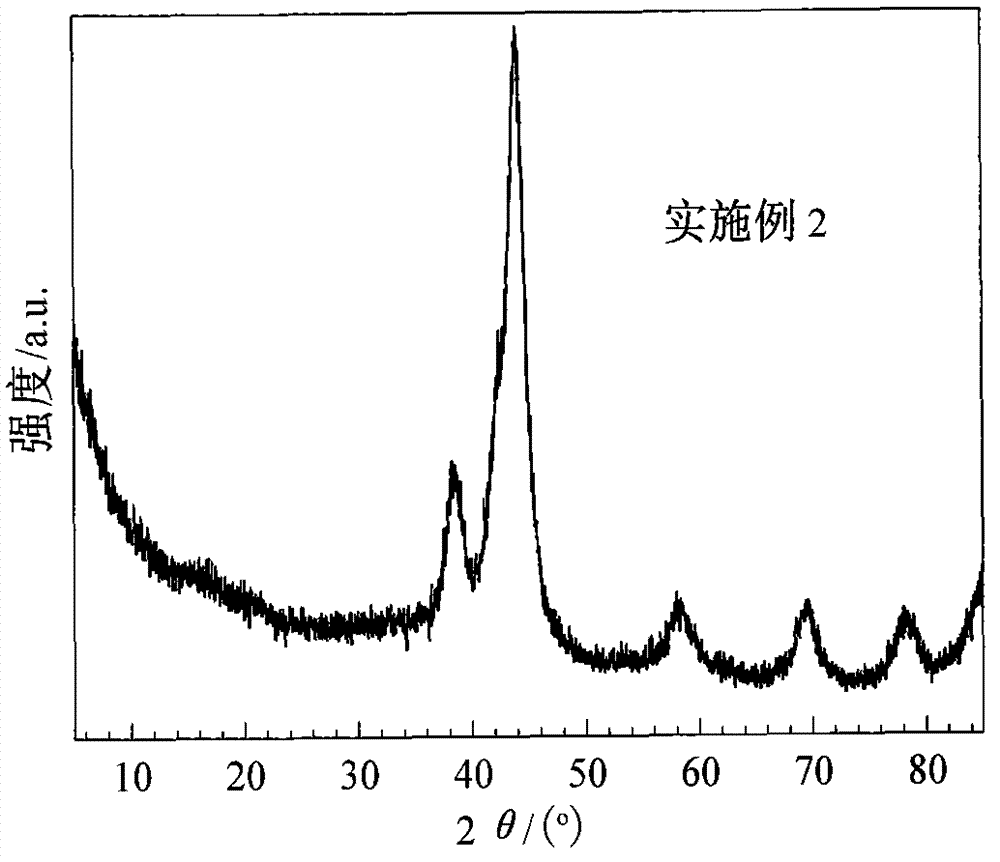
[0034] 0.0024g zinc oxide is changed into 0.0029g zinc hydroxide of equivalent substance amount, and other conditions are with embodiment 1. The XRD spectrum of the catalyst is shown in figure 2 , it can be seen that it is similar to the XRD spectrum of the catalyst in Example 1, and the diffraction peaks at 38.3°, 43.8°, 58.2°, 69.4° and 78.1° in the figure all belong to the characteristic peaks of crystalline metal Ru. It can be seen that the catalyst is in a crystalline state, and the Ru crystallite size is 4.4nh as measured by the FWHM method. Inductively coupled plasma atomic emission spectrometry measured that the mass percentage of Zn in the catalyst was 0.31%, and the mass percentage of B was 0.57%.
Embodiment 3
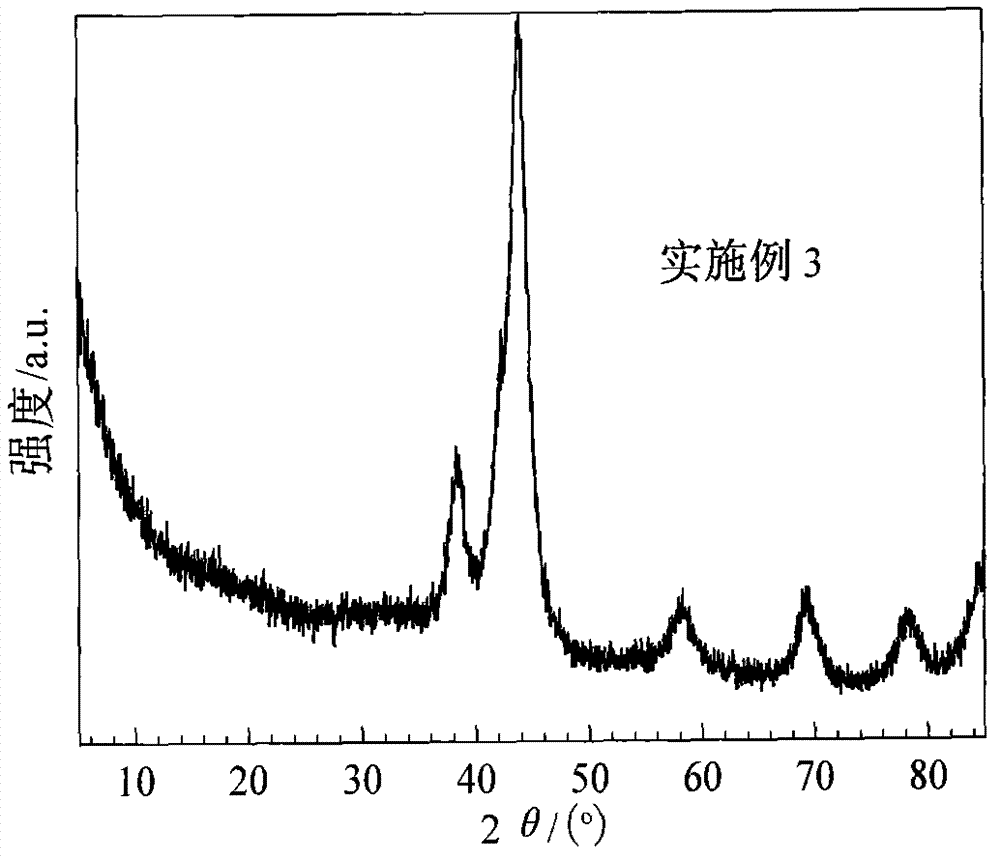
[0036]Change 0.0242g boric acid and 0.0024g zinc oxide into 0.0197g borax and 0.0085g zinc sulfate heptahydrate, and other conditions are with embodiment 1. The XRD spectrum of the catalyst is shown in image 3 , it can be seen that it is similar to the XRD spectrum of the catalyst in Example 1, and the diffraction peaks at 38.3°, 43.8°, 58.2°, 69.4° and 78.1° in the figure all belong to the characteristic peaks of crystalline metal Ru. The Ru crystallite size was measured to be 4.2nh by FWHM method. The mass percentage of Zn in the catalyst was measured by inductively coupled plasma atomic emission spectrometry to be 0.32%, and the mass percentage of B was 0.58%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com