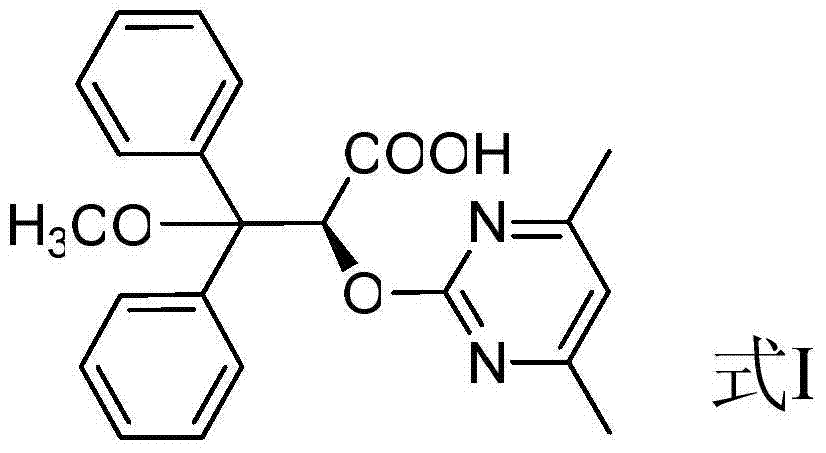
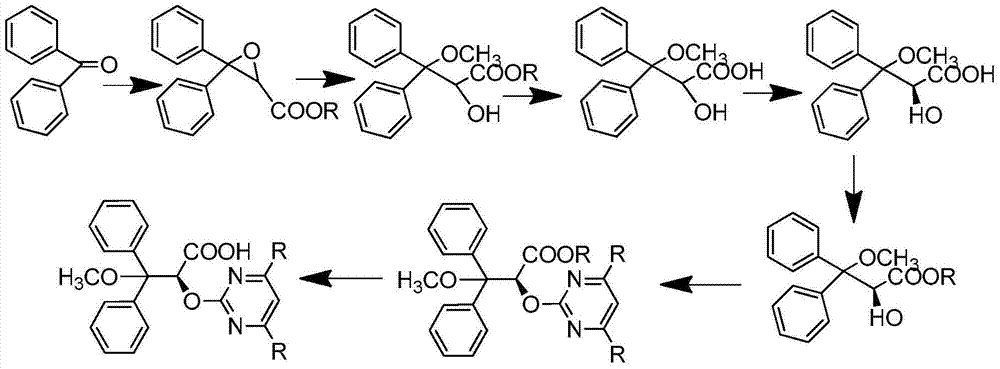
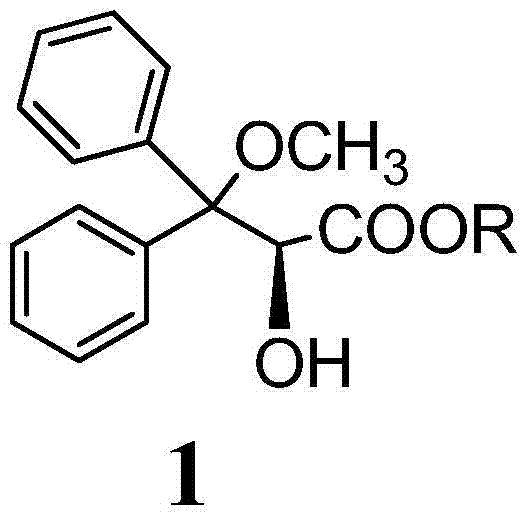
Preparation method for ambrisentan intermediate compound
An ambesentan and compound technology, which is applied in the field of preparation of ambesentan intermediates, can solve the problems of low yield of final products, cannot be produced on a large scale, complex synthesis steps, etc., and achieves simple preparation method, low cost, and reaction yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound 10, wherein R is methyl:
[0034]
[0035] Dissolve the mixture of 10g benzophenone and 7.1g ethyl chloroacetate in 100ml tetrahydrofuran, add 5.9g sodium methylate and stir for 90 minutes at -4°C, continue stirring for 30 minutes at -10°C--5°C, and then add to 25-35°C, extracted with water and ethyl acetate, washed the organic layer with saline solution, then dried over anhydrous sodium sulfate, and distilled under reduced pressure at 60°C to obtain 13g of the product (compound 10), with a yield of 93%.
Embodiment 2
[0037] Preparation of compound 10, wherein R is methyl:
[0038]
[0039] Dissolve the mixture of 100g benzophenone and 71g ethyl chloroacetate in 1000ml tetrahydrofuran, add 59g sodium methylate and stir for 90 minutes at -4°C, continue stirring for 30 minutes at -10°C--5°C, then add to 25°C -35°C, extracted with water and ethyl acetate, washed the organic layer with saline solution, then dried over anhydrous sodium sulfate, and distilled under reduced pressure at 60°C to obtain 129.8g of the product (compound 10), with a yield of 93%.
Embodiment 3
[0041] Preparation of compound 1, wherein R is methyl:
[0042]
[0043] Dissolve 10g of compound 10 in 20ml of methanol, then add 3.9g of sulfonic acid, stir at 25-35°C for two hours, then add 25g of tris(ethylenediamine) cobalt salt, stir at 55-60°C for 45 minutes, then cool Continue to stir for 1 hour to 25-35 ℃, have solid to separate out, filter, wash with methyl tert-butyl ether, filter, dry, obtain product 4.86g (compound 1), yield is 43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com