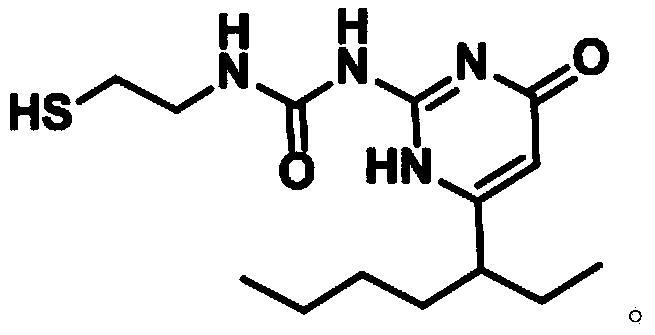
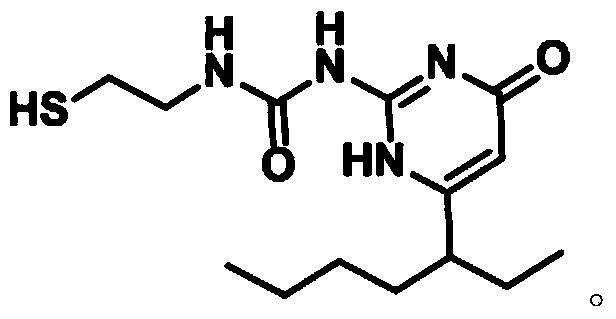
Compound as well as synthesis thereof and method for synthesizing double-UPy substituted compound by using same
A technology of compounds and sulfur compounds, which is applied in the field of compounds, their synthesis and the synthesis of double UPy substituted compounds, can solve the problems of increasing the burden of double UPy substituted compounds, restricting the development of supramolecular materials, and the difficulty of synthesizing aromatic compounds, etc., to achieve synthesis The method is scientific and reasonable, the scope of application is wide, and the effect of raw materials is easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
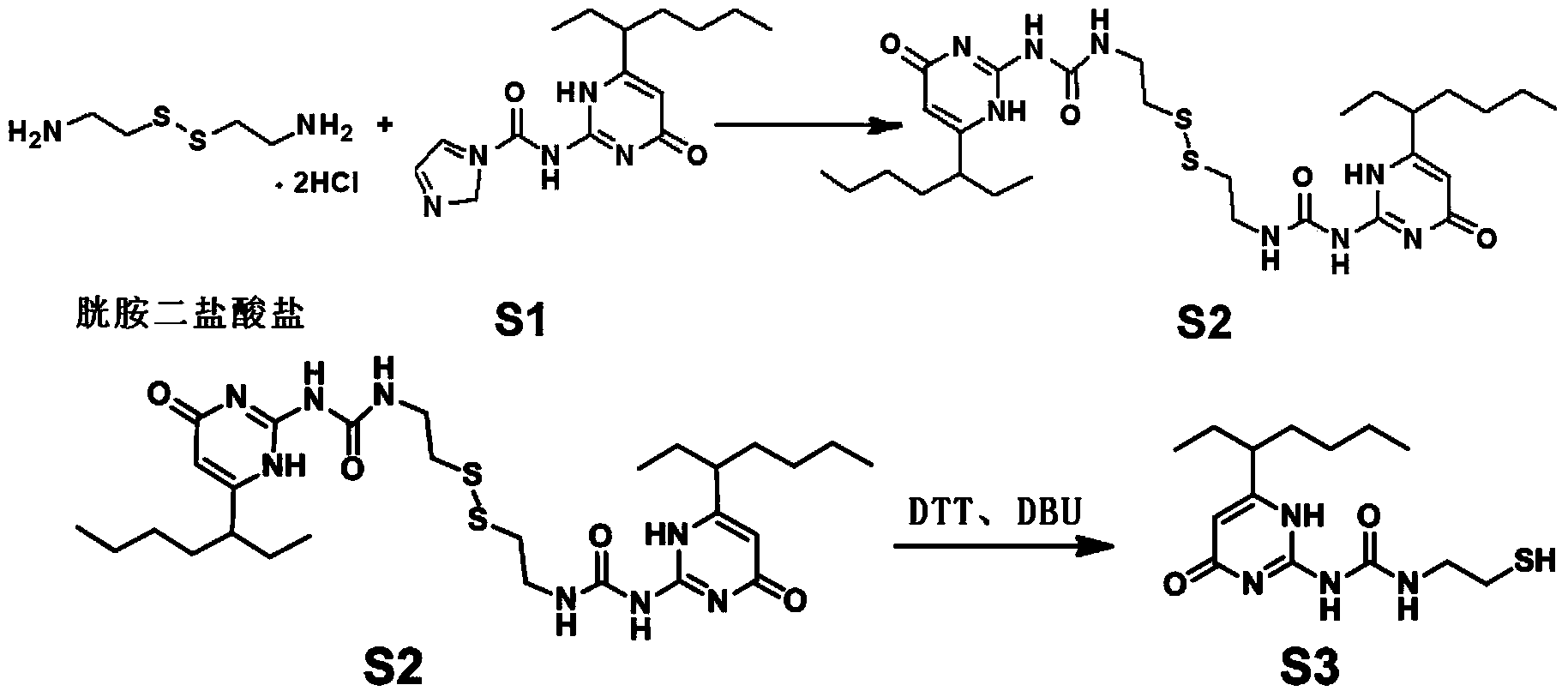
[0039] 1) Dissolve 224mg, 1mmol of cystamine dihydrochloride in 5-50mL of anhydrous dichloromethane, add compound S1 (456-912mg, 1.5-3mmol), stir at room temperature for 5-15h, wash with brine three times , the collected organic phase was spin-dried, and separated by column chromatography to obtain compound S2.
[0040] 2) Dissolve compound S2 (100 mg, 0.16 mmol) in 15-100 mL of anhydrous dichloromethane, add 37-248 mg (0.24-1.6 mmol) of 1,4-dithiothreitol, and add a catalytic amount of 1,8 - Diazabicyclo[5.4.0]undec-7-ene (DBU) was refluxed for 0.25-3 hours, washed with water, the organic phase was collected, and spin-dried to obtain the product S3.
[0041]
Embodiment 2
[0043] Take 7 mg of propynyl alcohol and 398 mg of compound S, add the initiator DMPA (2-16 mg), dissolve and mix with 1.2 mL of 1,2-dichloroethane, and then carry out the light reaction. After 2 hours of light, the reaction is complete, and the organic solvent is spin-dried in vacuum for 1, 2-dichloroethane, compound 1a was obtained by column chromatography. Isolated yield 97%. The NMR data of the compound 1a are as follows: 1 H NMR (CDCl 3 ,400MHz,ppm): δ13.17-13.09 (m, 2H), 11.99-11.95 (m, 2H), 10.43 (s, 2H), 5.84 (s, 2H), 3.86-3.40 (m, 6H), 2.97 -2.78 (m, 7H), 2.31 (s, 2H), 1.71-1.52 (m, 8H), 1.32-1.20 (m, 8H), 1.21-0.84 (m, 12H).
[0044]
Embodiment 3
[0046] 1) Synthesis of propynyl pyrenecarboxylate: 1-pyrenecarboxylic acid (372mg, 1.5mmol), propynyl alcohol (265-530mg, 2.25-4.5mmol), and potassium carbonate (1-10g, 7.5-75mmol) were added After reacting in 10-60mL N,N-dimethylformamide solvent at 80°C for 5-12h, add a large amount of ethyl acetate and wash with water, collect the organic phase, spin dry, and obtain 391mg of the product by column chromatography. The NMR data of this compound are expressed as follows: 1 H NMR (CDCl 3 ,400MHz,ppm):δ9.32(d,J=9.2Hz,1H),8.71(d,J=8.4Hz,1H),8.30(q,3H),8.22(t,2H),8.12(q, 2H), 5.12(d, J=2.4Hz, 2H), 2.59(t, 1H).
[0047]2) Take 15 mg of propynyl pyrenecarboxylate and 398 mg of compound S in 1), add the initiator DMPA (2-16 mg), dissolve and mix with 1.2 mL of 1,2-dichloroethane, then carry out light reaction, and react after 2 hours of light After completion, the organic solvent 1,2-dichloroethane was spin-dried in vacuum, and compound 1b was obtained by column chromatography. Is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com