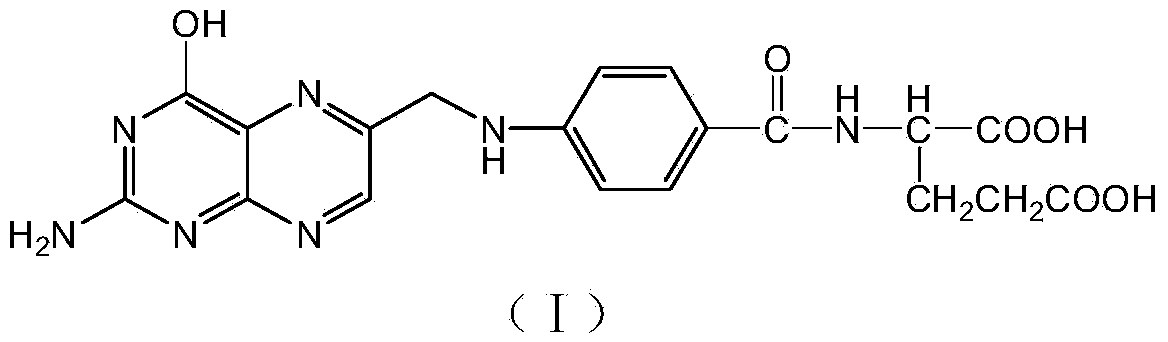
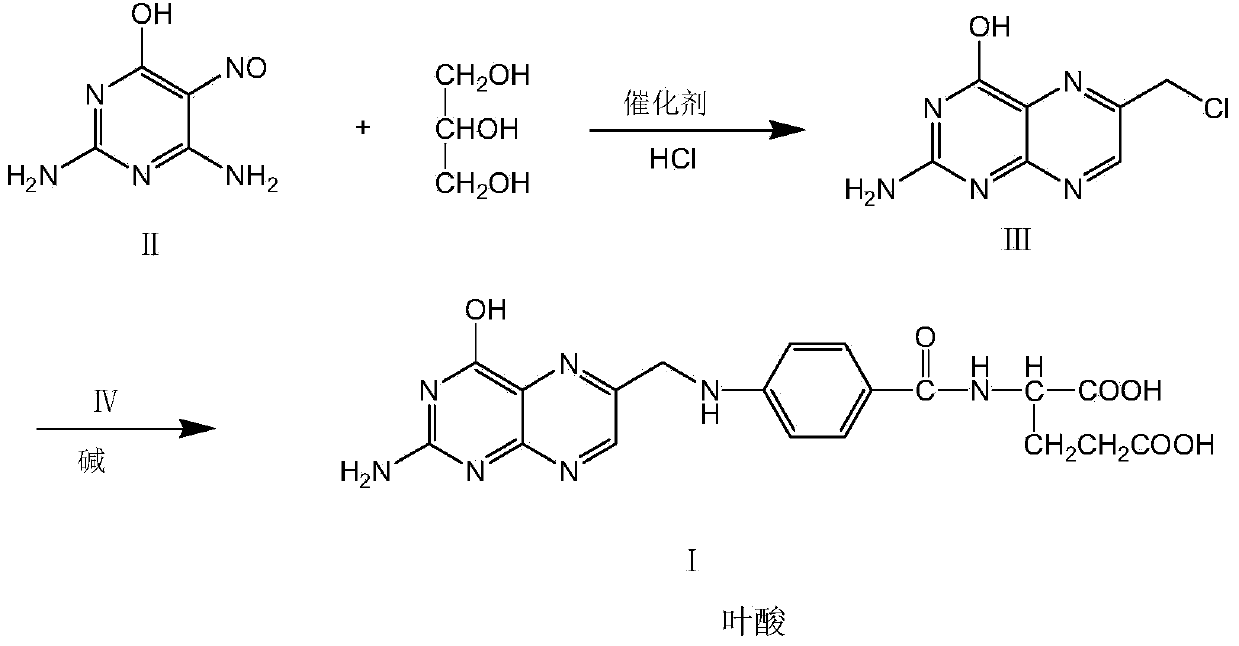
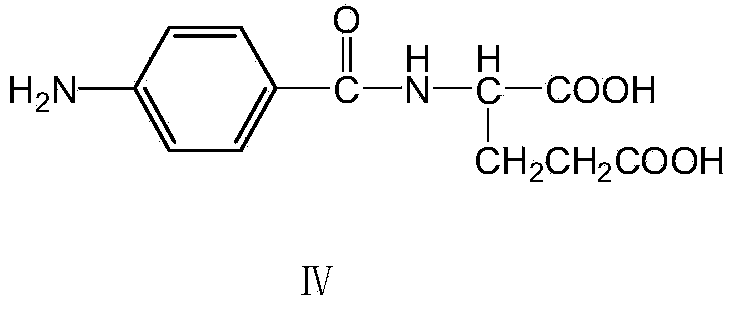
Environment-friendly preparation method of synthetic folic acid
A technology of folic acid and environmental protection, applied in the direction of organic chemistry, etc., can solve the problems of environmental pollution, no industrial value, not easy to obtain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of folic acid
[0044] Add 250 g of 1,2-dichloroethane, 15 g of 90% sulfuric acid, 5.2 g of cuprous chloride, and 46 G (0.5 mole) glycerin, 30.2 g (0.2 mole) 2,4-diamino-5-nitroso-6-hydroxypyrimidine, under nitrogen protection, reflux to remove water for 7-9 hours (approximately 16-17 gram). Cool to room temperature 20°C, add 0.6 g of tetrabutylammonium chloride, 32 g of 35% concentrated hydrochloric acid, stir and reflux at 20°C for 3 hours. 2-Amino-4-hydroxy-6-chloromethylpteridine (III) is produced. Adjust the pH value to 5-6 with 20% sodium hydroxide at 20°C, add 50.5 g (0.19 moles) of p-aminobenzoyl-L-glutamic acid, raise the temperature to 40-45°C and stir for 4 hours, while adding 20% sodium hydroxide aqueous solution keeps the pH value at 5-6, and 2-amino-4-hydroxy-6-chloromethylpteridine is condensed with p-aminobenzoyl-L-glutamic acid to prepare folic acid. After the reaction is completed, cool to 20°C, pour the reaction liqui...
Embodiment 2
[0045] Embodiment 2: the preparation of folic acid
[0046] In a flask equipped with agitator, thermometer, nitrogen conduit, water trap with cross connector and reflux condenser, add 260 g of chlorobenzene, 15 g of 90% sulfuric acid, 6.8 g of zinc chloride dihydrate, 36.8 g (0.4 mol) Glycerin, 30.2 g (0.2 mol) of 2,4-diamino-5-nitroso-6-hydroxypyrimidine, under nitrogen protection, reflux to remove water for 11 hours (approximately 16-17 g of water was separated). Cool to room temperature 20°C, add 0.6 g of tetrabutylammonium chloride, 32 g of 35% concentrated hydrochloric acid, and stir at 20°C for 3 hours. Adjust the pH value to 5-6 with 20% sodium hydroxide at 20°C, add 53.0 g (0.2 moles) of p-aminobenzoyl-L-glutamic acid, raise the temperature to 40-45°C and stir for 4 hours, while adding 20% sodium hydroxide aqueous solution maintains a pH value of 5-6. After the reaction is completed, cool to 20°C, pour the reaction liquid into 450 grams of water, filter (recover the ...
Embodiment 3
[0047] Embodiment 3: the preparation of folic acid
[0048] In a flask equipped with stirring, thermometer, nitrogen conduit, water separator and reflux condenser, add 250 g of 2-methyltetrahydrofuran, 21 g of 75% phosphoric acid, 11 g of zinc chloride dihydrate, 36.8 g (0.4 mol) Glycerin, 30.2 g (0.2 mol) of 2,4-diamino-5-nitroso-6-hydroxypyrimidine, under nitrogen protection, reflux to remove water for 11 hours (approximately 16-17 g of water was separated). Cool to room temperature 20°C, add 0.6 g of tetrabutylammonium chloride, 32 g of 35% concentrated hydrochloric acid, and stir at 20°C for 3 hours. Adjust the pH value to 5-6 with 20% sodium hydroxide at 20°C, add 53.0 g (0.2 moles) of p-aminobenzoyl-L-glutamic acid, raise the temperature to 40-45°C and stir for 4 hours, while adding 20% sodium hydroxide aqueous solution maintains a pH value of 5-6. After the reaction is completed, cool to 20°C, pour the reaction liquid into 450 grams of water, filter (recover the solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com