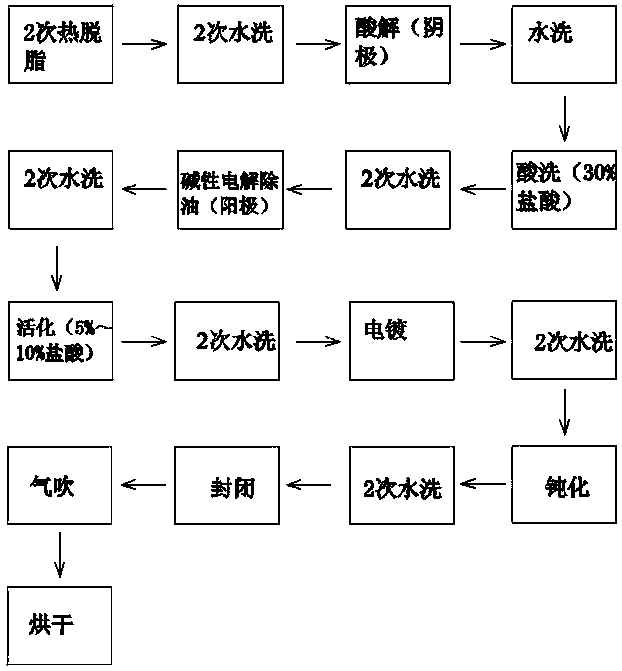
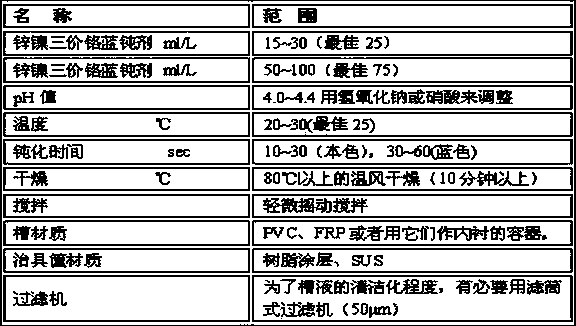
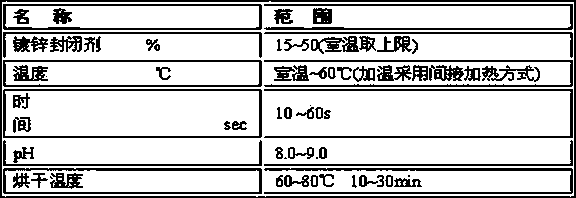
Zinc-tin alloy electroplating method
An alloy electroplating and alloy electroplating solution technology, applied in the field of zinc-tin alloy electroplating, can solve the problems of lack of brightness, increase the operation process, affect the quality of the plating solution, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] Below by embodiment the present invention is described in further detail:
[0039] SnSO 4 35g / L
[0040] ZnSO 4 ·7H 2 o 40g / L
[0041] Citric acid 100g / L
[0042] Benzylidene acetone 0.1g / L
[0043] Ten Polyglycerol 100g / L
[0044] 1.3 ureide quaternary ammonium polymer 1g / L
[0045] Ascorbic acid 5g / L
[0046] Sodium Lauryl Sulfate 80g / L
[0047] Amphoteric surfactant imidazoline 0.3~15g / L
[0048] Polyethyleneimine 0.5g / L
[0049] Among them, the role of citric acid as a complexing agent: due to the standard electrode potential difference between metals, Sn 2+ The standard electrode potential is equal to -0.14V, Zn 2+ The standard electrode potential of the standard electrode is equal to -0.763V. The difference between the two is large. It is difficult to form a co-lamination layer in the plating solution. It is necessary to bring the deposition potentials of the two closer together. On the other hand, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com