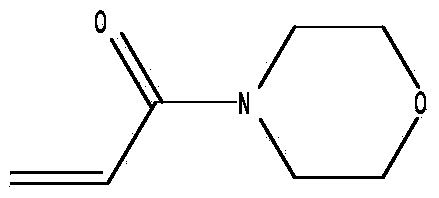
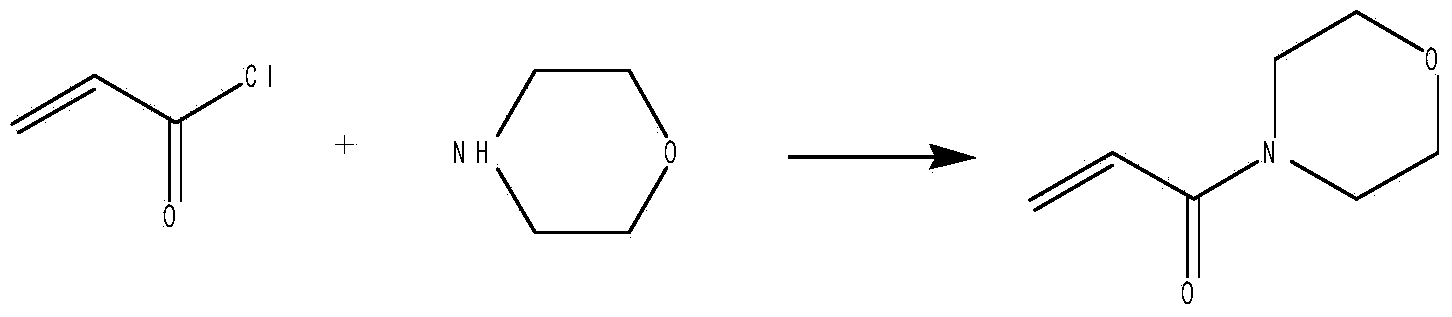
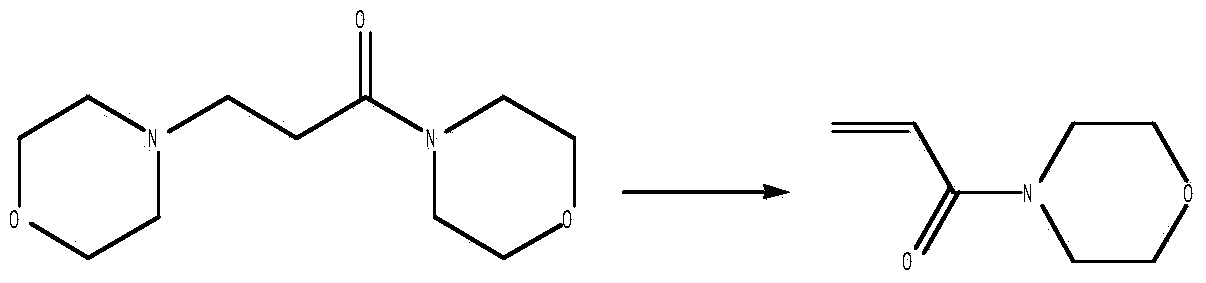
Synthetic improvement method of acryloylmorpholine
A technology based on acryloylmorpholine and morpholino, applied in the field of organic synthesis, can solve problems such as difficult separation of acryloylmorpholine, difficult control of thermal cracking reaction, serious thermal polymerization of products, etc., to achieve easy separation of products and excellent reaction conditions Ease of control and less effect of aggregation phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 6.00 g (0.070 mol) of methyl acrylate, 5.11 g (0.07 mol) of diethylamine, and 0.30 g (0.0024 mol) of p-hydroxyanisole into a 100 mL four-neck flask, and keep the temperature at 65°C to 68°C for 1 hour After finishing the reaction, the reaction solution was evaporated under reduced pressure to obtain 8.85g (0.060mol) of methyl 3-methoxy-diethylpropionate, and 6.26g (0.072mol) of morpholine and 0.32g (0.006mol) of sodium methoxide were added , keep the temperature at 80°C to 85°C, react for 5 to 8 hours, after the end, add hydrochloric acid to neutral, and rotary evaporate under reduced pressure to obtain the crude product 3-morpholinyl-diethylpropionamide, then add methanol to dissolve, and filter with suction In addition to sodium chloride, the mother liquor was rotary evaporated under reduced pressure to obtain 7.20 g of 3-morpholino-diethylpropionamide. Continue to add 0.30 g (0.0024 mol) of polymerization inhibitor, and distill under reduced pressure to obtain 3....
Embodiment 2
[0041] Add 6.00 g (0.070 mol) of methyl acrylate, 10.22 g (0.14 mol) of diethylamine, and 0.30 g (0.0024 mol) of p-hydroxyanisole into a 100 mL four-neck flask, and keep the temperature at 65°C to 68°C for 1 hour After finishing the reaction, the reaction solution was evaporated under reduced pressure to obtain 9.03g (0.062mol) of methyl 3-methoxy-diethylpropionate, and 10.79g (0.124mol) of morpholine and 3.35g (0.062mol) of sodium methoxide were added , keep the temperature at 80°C to 85°C, react for 5 to 8 hours, after the end, add hydrochloric acid to neutral, and rotary evaporate under reduced pressure to obtain the crude product 3-morpholinyl-diethylpropionamide, then add methanol to dissolve, and filter with suction In addition to sodium chloride, the mother liquor was rotary evaporated under reduced pressure to obtain 7.53 g of 3-morpholino-diethylpropionamide. Continue to add 0.30 g (0.0024 mol) of polymerization inhibitor, and distill under reduced pressure to obtain ...
Embodiment 3
[0043] Add 6.00 g (0.070 mol) of methyl acrylate, 7.66 g (0.10 mol) of diethylamine, and 0.30 g (0.0024 mol) of p-hydroxyanisole into a 100 mL four-neck flask, and keep the temperature at 65°C to 68°C for 1 hour After finishing the reaction, the reaction solution was evaporated under reduced pressure to obtain 8.83g (0.061mol) of methyl 3-methoxy-diethylpropionate, and 6.35g (0.073mol) of morpholine and 1.65g (0.031mol) of sodium methoxide were added , keep the temperature at 80°C to 85°C, react for 5 to 8 hours, after the end, add hydrochloric acid to neutral, and rotary evaporate under reduced pressure to obtain the crude product 3-morpholinyl-diethylpropionamide, then add methanol to dissolve, and filter with suction In addition to sodium chloride, the mother liquor was rotary evaporated under reduced pressure to obtain 7.82 g of 3-morpholino-diethylpropionamide. Continue to add 0.30 g (0.0024 mol) of polymerization inhibitor, and distill under reduced pressure to obtain 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com