A kind of furanone aniline derivative prepared by metronidazole and its preparation method and application in antibacterial drugs
A technology of furanone aniline and its derivatives, applied in antibacterial drugs, organic chemistry, etc., to achieve good inhibitory and killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
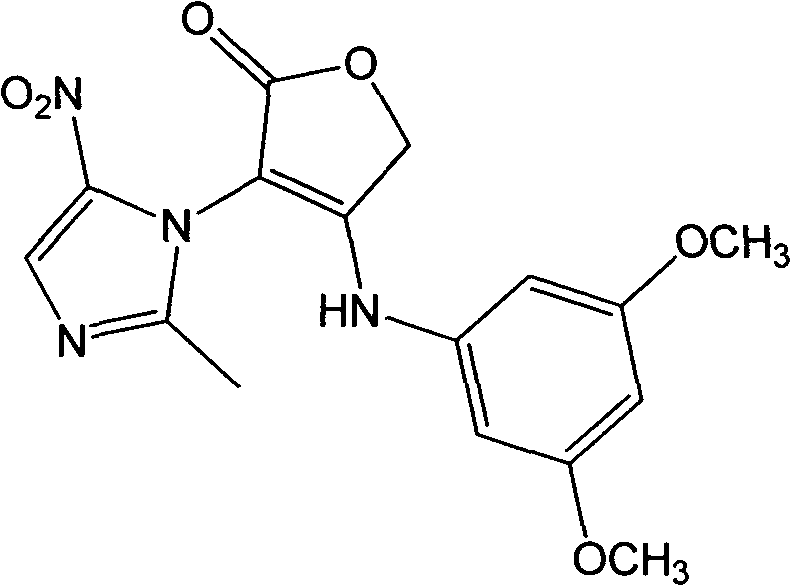
[0019] Example 1: 4-((3,5-dimethoxyphenyl)amino)-3-(2-methyl-5-nitro-1H-imidazol-1-yl)furan-2(5H)- Preparation of Ketone (Compound 1)
[0020]
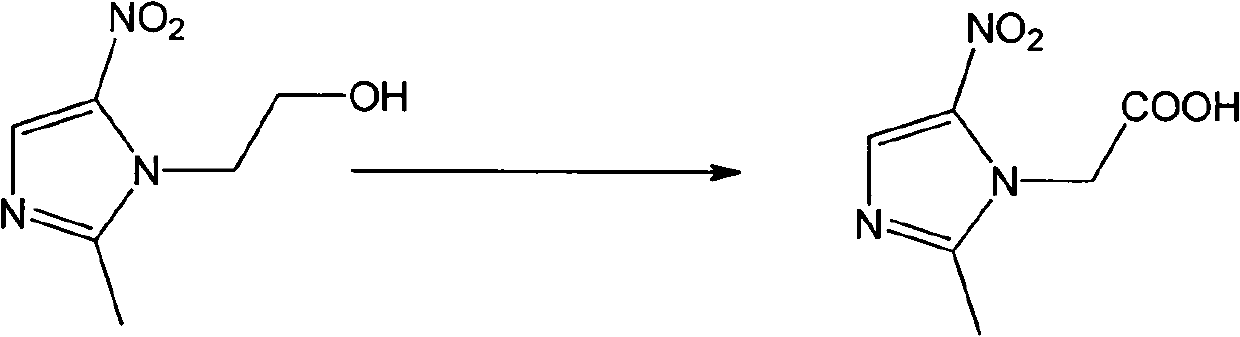
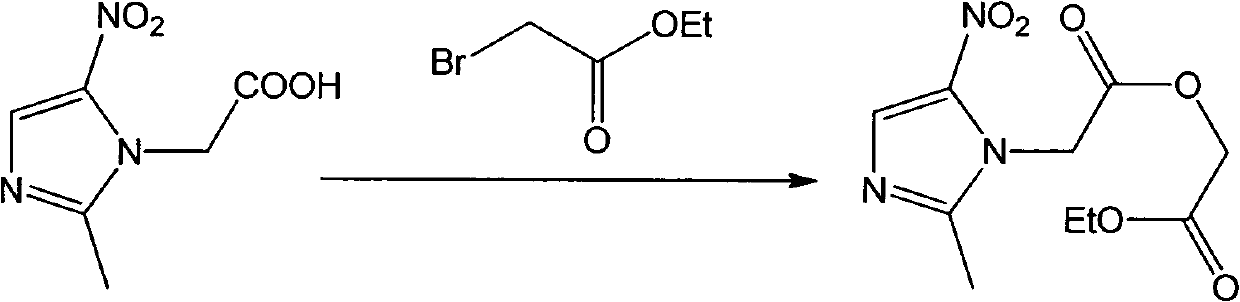
[0021] Dissolve 5mmol of metronidazole in 100mL of water, add 10mmol of sodium dichromate, stir magnetically to mix evenly, slowly drop in 98% concentrated sulfuric acid, and react at room temperature for 5h (TLC detects the progress of the reaction). After the reaction, dilute NaOH solution Adjust the pH to 9.1, a large amount of solids are produced, filter the solids, adjust the pH of the filtrate to 4.6 with dilute hydrochloric acid, extract 3 times with ethyl acetate, 100 mL each time, take the organic phase, and evaporate all the solvents in vacuo to obtain an intermediate product; Dissolve 3mmol of the above product and 9mmol of sodium ethoxide in 50mL of absolute ethanol, add 6mmol of ethyl bromoacetate at room temperature, raise the temperature to 45°C and react for 10h (TLC detection). After the reaction, filter with sucti...
Embodiment 2
[0022] Embodiment two: the antibacterial activity of compound
[0023] Bacteria were suspended in MH medium at a concentration of approximately 10 5 cfu / mL, the bacterial solution was added to a 96-well plate (100 μL of bacterial solution per well), the culture medium was used as a blank control, DMSO was used as a negative control instead of the test substance, and kanamycin was used as a positive control. Dissolve the test substance in DMSO to make 1600, 800, 400, 200, 100, 50 μg / mL solutions respectively (for MIC 50 If it is less than 5 μg / mL, in further experiments, the prepared concentration gradient is 100, 50, 25, 12.5, 6.25 μg / mL), and added to the 96-well plate in an amount of 11 μL per well [the final concentration of the drug solution is 160 , 80, 40, 20, 10, 5 μg / mL (10, 5, 2.5, 1.25, 0.63 μg / mL for the latter)], four parallel experiments were done for each concentration gradient. Put the 96-well plate in an incubator at 37°C for 24 h, then add 25 μL per mL of PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com