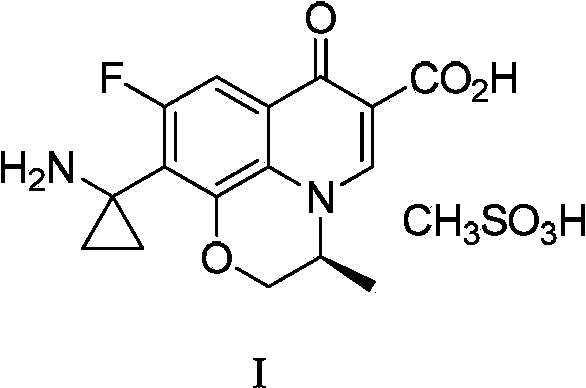
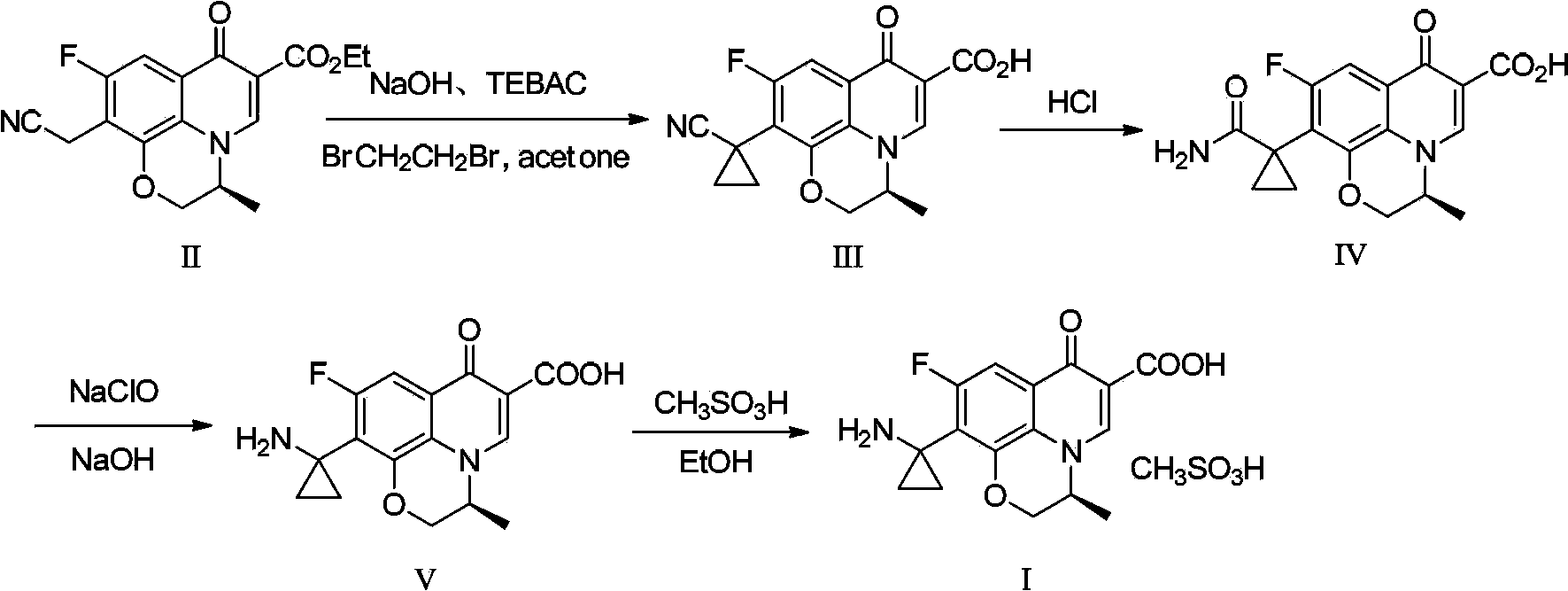
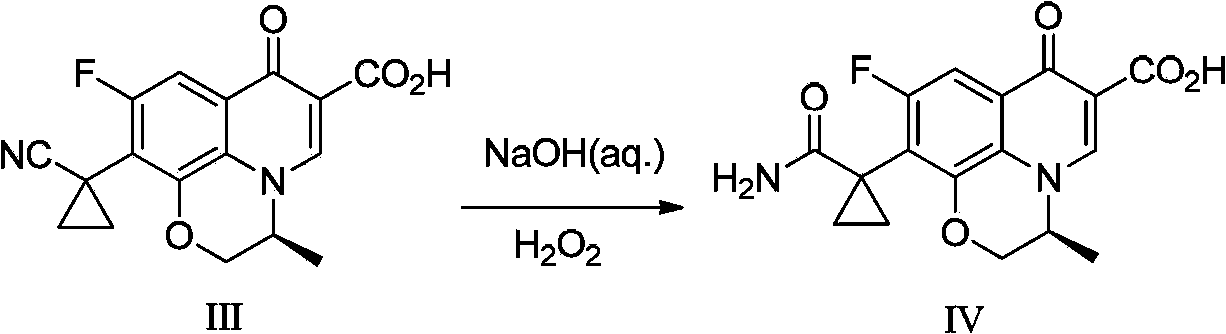Preparation method of pazufloxacin intermediate
A technology of pazufloxacin and intermediates, which is applied in the field of drug synthesis, can solve the problems of low yield, high pressure on environmental protection, and high potential safety hazards, and achieve the effects of high yield, low pressure on environmental protection, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0035]In a 250mL three-necked flask, add lye made of 20g of sodium hydroxide and 100mL of water, lower the temperature of the reaction system to 0~5°C while stirring, add 3g of benzyltriethylammonium chloride, and stir for 1 hour; Add 10 g of raw material compound II ((s)-10-ethylcyano-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyridine[1,2 ,3-de][1,4]Benzoxazine-6-carboxylate ethyl ester, commercially available), stirred at about 5°C for 1 hour, added 50mL of acetone and 4.5g of 1,2-dichloroethyl After the addition, the reaction system was stirred and reacted at room temperature for 5 hours, then heated to reflux for 6 hours, and then cooled. When the temperature of the system was between 20 and 30°C, it was neutralized by adding half-concentration hydrochloric acid dropwise to pH After = 6, the reaction system becomes turbid, and a large amount of solids precipitate out. After stirring for 10 minutes, the pH value is re-measured to be stable. After cooling down to 10-20°C, conti...
preparation Embodiment 2
[0037] In a 250mL three-necked flask, add lye made of 20g of sodium hydroxide and 100mL of water, lower the temperature of the reaction system to 0~5°C while stirring, add 3g of benzyltriethylammonium chloride, and stir for 1 hour; Add 10g of raw material compound II at 0~5°C, stir at about 5°C for 1 hour, add 50mL of a mixed solution of 4-methyl-2-pentanone and 4.5g of 1,2-dichloroethane, and complete the addition , the reaction system was stirred and reacted at room temperature for 5 hours, then heated to reflux for 3 hours, and then lowered the temperature. When the temperature of the system was between 20 and 30°C, it was neutralized by adding half-concentration hydrochloric acid to pH=6 or so, and the reaction system became It was turbid and a large amount of solids were precipitated. After stirring for 10 minutes, the pH value was re-measured to be stable. After cooling down to 10-20°C, stirring was continued for 1 hour, and the white solid was filtered to obtain compound...
preparation Embodiment 3
[0039] In a 250mL three-necked flask, add lye made up of 15g of sodium hydroxide and 150mL of water, lower the temperature of the reaction system to 0~5°C while stirring, add 3g of benzyltriethylammonium chloride, and stir for 1 hour; Add 10g of raw material compound II at 0~5°C, stir at about 5°C for 1 hour, add 4.5g of a mixed solution composed of 1,2-dichloroethane, after the addition is complete, the reaction system is stirred at room temperature for 5 hours, and then heated After 2 hours of reflux reaction, lower the temperature. When the temperature of the system is between 20 and 30°C, add half-concentration hydrochloric acid to neutralize it dropwise. When the pH is about 6, the reaction system becomes turbid and a large amount of solids are precipitated. After stirring for 10 minutes, resume The pH value was measured to be stable, and the temperature was lowered to 10-20°C, and the stirring was continued for 1 hour, and the white solid was filtered to obtain compound I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com