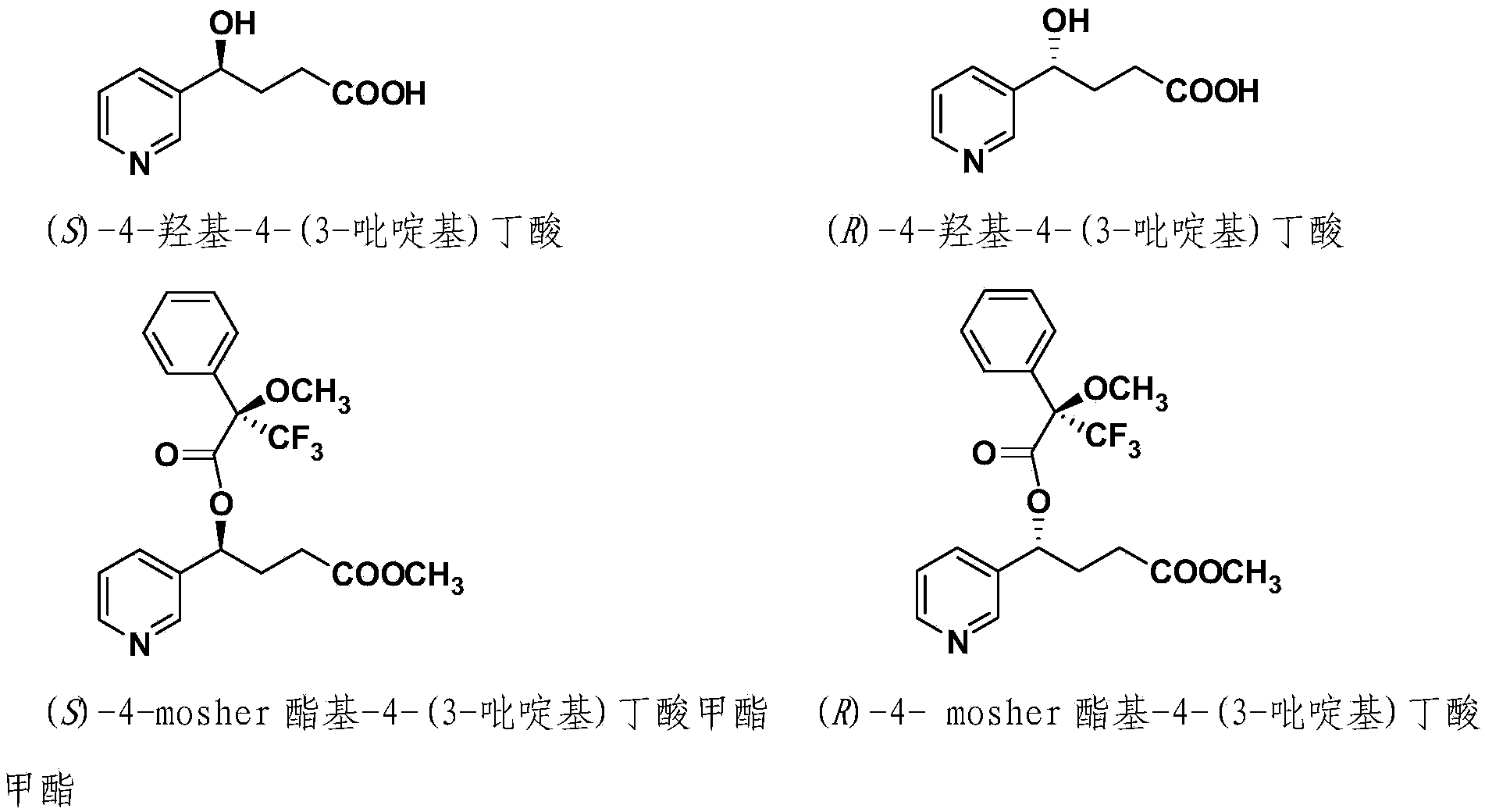
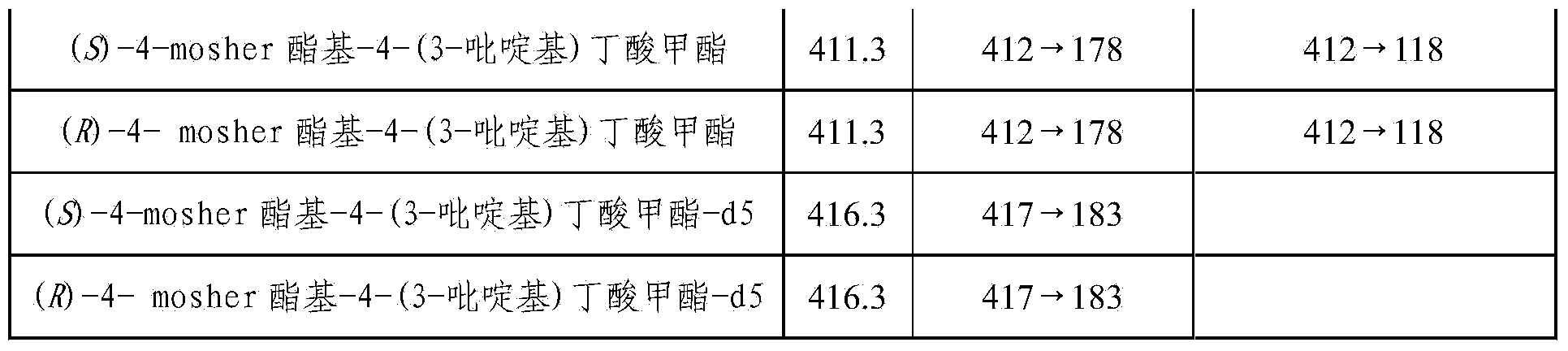
Method for detecting 4-hydroxyl-4-(3-pyridyl) butyric acid in urine
A technology based on pyridyl and hydroxyl, which is applied in the field of physical and chemical testing of tobacco alkaloids, and achieves the effects of good stability, low detection limit, and easy popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]1. Chemical reagent preparation: (S)-(+)-α-methoxy-α-trifluoromethylphenylacetyl chloride (CAS No. 20445-33-4), (S)-(+)-α-- Methoxy-(trifluoromethyl)phenylacetic anhydride (CAS No. 85541-57-7), (±)-4-hydroxy-4-(3-pyridyl)butanoic acid (CAS No. 15569-97-8) , (±)-4-Hydroxy-4-(3-pyridyl)butanoic acid-d5, triethylamine, diazomethane, dimethyl sulfate.
[0033] 2. Derivatization of (±)-4-hydroxy-4-(3-pyridyl)butanoic acid and establishment of standard curve:
[0034] Using anhydrous acetonitrile as solvent, prepare (±) 4-hydroxy-4-(3-pyridyl) butyric acid standard single standard solution with a concentration of 1 μg / mL, keep four significant figures; use anhydrous acetonitrile in the same way Prepare a single standard solution of (±)-4-hydroxy-4-(3-pyridyl)butanoic acid-d5 with a concentration of 1 μg / mL as the solvent; use anhydrous acetonitrile as the solvent, prepare the solution containing (±) according to Table 2 - Mixed standard solution of 4-hydroxy-4-(3-pyridyl)but...
Embodiment 2
[0044] Repeat Example 1, with the following differences:
[0045] 1. Derivatization of (±)-4-hydroxy-4-(3-pyridyl)butanoic acid and establishment of standard curve
[0046] Using anhydrous acetonitrile as a solvent, prepare a single standard solution of (±)-4-hydroxy-4-(3-pyridyl)butyric acid standard with a concentration of 1 μg / mL, and keep four significant figures; Acetonitrile was used as the solvent to prepare a single standard solution of (±)-4-hydroxy-4-(3-pyridyl)butyric acid-d5 with a concentration of 1 μg / mL; anhydrous acetonitrile was used as the solvent to prepare the solution containing (±) )-4-hydroxy-4-(3-pyridyl)butanoic acid and deuterated (±)-4-hydroxy-4-(3-pyridyl)butyric acid-d5 mixed standard solution; the prepared concentration is 1μg / mL (S)-(+)-α-methoxy-α-trifluoromethylphenylacetic anhydride in anhydrous acetonitrile solution; prepare a solution of triethylamine in anhydrous acetonitrile with a concentration of 1 μg / mL; take 2 mL respectively Mix the...
Embodiment 3
[0050] Repeat Example 1, with the following differences:
[0051] 1. Derivatization of (±)-4-hydroxy-4-(3-pyridyl)butanoic acid and establishment of standard curve
[0052] Using anhydrous acetonitrile as a solvent, prepare a single standard solution of (±)-4-hydroxy-4-(3-pyridyl)butyric acid standard with a concentration of 1 μg / mL, and keep four significant figures; Acetonitrile was used as the solvent to prepare a single standard solution of (±)-4-hydroxy-4-(3-pyridyl)butyric acid-d5 with a concentration of 1 μg / mL; anhydrous acetonitrile was used as the solvent to prepare the solution containing (±) )-4-hydroxy-4-(3-pyridyl)butanoic acid and deuterated (±)-4-hydroxy-4-(3-pyridyl)butyric acid-d5 mixed standard solution; the prepared concentration is 1μg / mL Anhydrous acetonitrile solution of (S)-(+)-α-methoxy-α-trifluoromethylphenylacetyl chloride; prepare anhydrous acetonitrile solution of triethylamine with a concentration of 1 μg / mL; take 0.5 Put the mixed standard solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com