A calcium preparation, a preparing method thereof and applications thereof
A technology of calcium preparations and calcium salts, which is applied in the field of calcium supplementation, and can solve problems such as renal calcification, kidney stones, hypercalcemia, and increased hazards of kidney stones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of calcium preparation provided by the invention comprises steps:
[0034] In the first step, calcium salt is mixed with diluent to obtain mixture 1;
[0035] In the second step, the mixture 1 is subjected to wet granulation with a binder solution containing vitamin K to obtain primary granules;
[0036] In the third step, the vitamin D ethanol solution is sprayed on the primary particles to obtain secondary particles; and
[0037] In the fourth step, the secondary granules are mixed with flavoring agents, lubricants, and / or fillers to obtain the calcium preparation provided by the present invention.
[0038] The diluent described in the first step is selected from two or more of the following: glucose, starch, mannitol, sucrose, xylitol, lactose, dextrin, and microcrystalline cellulose.
[0039] The binder described in the second step is selected from one or more of the following: starch, glucose, povidone, and hydroxypropylmethylcellulose. In...
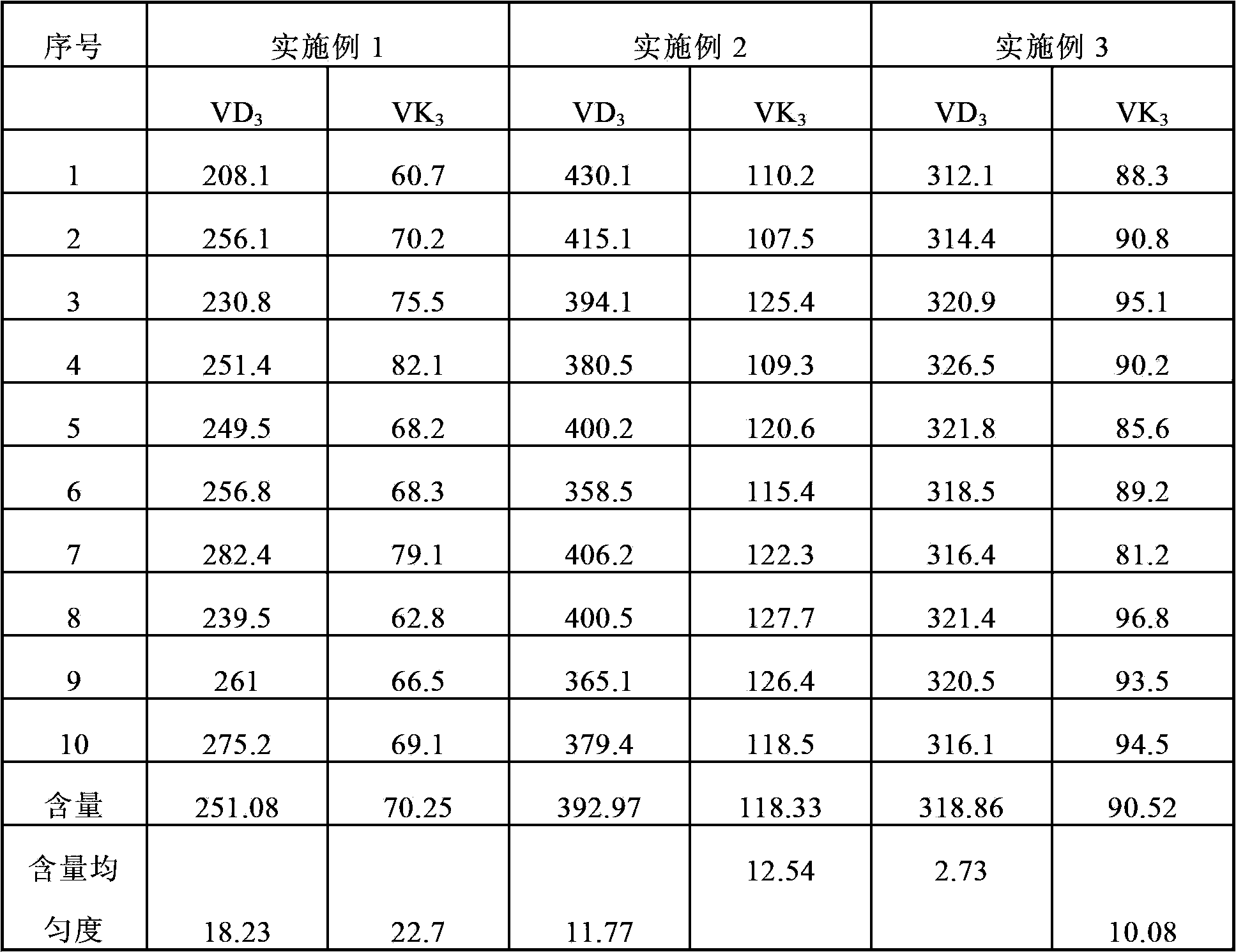
Embodiment 1
[0063] Preparation of vitamin DK calcium preparation 1 (quantity of 1000 tablets)
[0064] prescription:
[0065] raw material
Usage amount
1250
Vitamin D 3
6.5mg
Vitamin K 3
75mg
povidone
38g
glucose
50g
11.5g
[0066] Process steps:
[0067] Pass the prescribed amount of calcium carbonate, vitamin D, and vitamin K through a 100-mesh sieve, and pass the prescribed amount of povidone, glucose, and magnesium stearate through a 80-mesh sieve; dissolve the povidone in water at about 50°C, and stir for 30 Minutes to form a uniform binder solution for later use; put calcium carbonate, glucose, vitamin D, and vitamin K in a V-type mixer and mix evenly; put the mixed materials in a wet granulation pot to make Granules, 20-mesh sieve for granulation; put the granules into a fluidized bed, and dry at 50°C; mix the prepared granules with the prescrib...
Embodiment 2
[0069] Preparation of vitamin DK calcium preparation 2 (quantity of 1000 tablets)
[0070] prescription:
[0071] raw material
Usage amount
calcium carbonate
1250g
Vitamin D 3
10mg
120mg
povidone
35g
glucose
50g
11.5g
[0072] Process steps:
[0073] Pass the prescribed amount of calcium carbonate, vitamin D, and vitamin K through a 100-mesh sieve, and pass the prescribed amount of povidone, glucose, and magnesium stearate through a 80-mesh sieve; dissolve the povidone in water at about 50°C, and stir for 30 Minutes, to form a uniform adhesive solution, set aside; sieve and mix vitamin D, vitamin K and the same amount of glucose, and increase the amount in turn until all the glucose is mixed in, then put the mixture in a V-shaped mixer, add Equal amount of calcium carbonate, increasing in equal amounts in turn until all the calcium carbonate is m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com