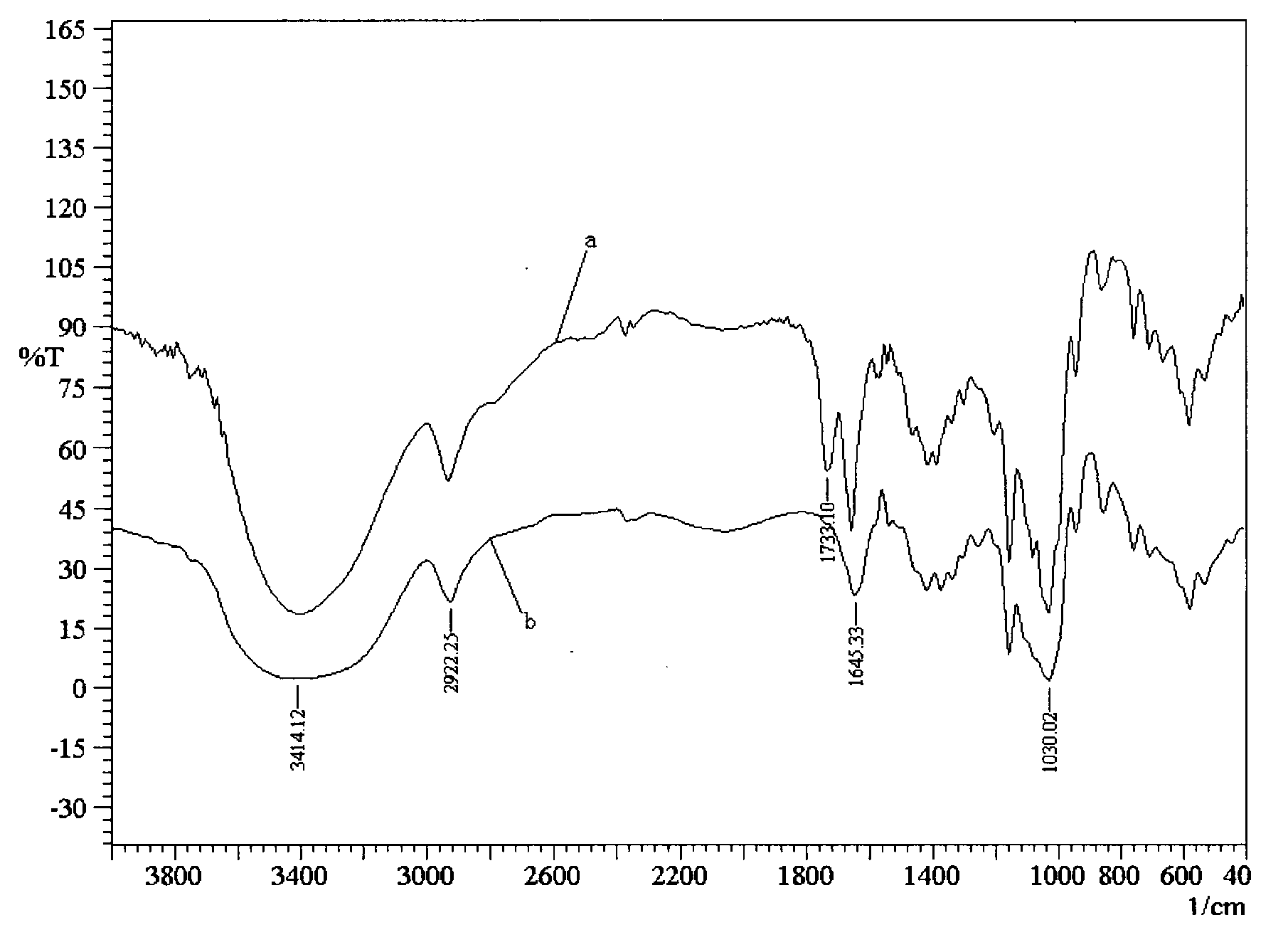
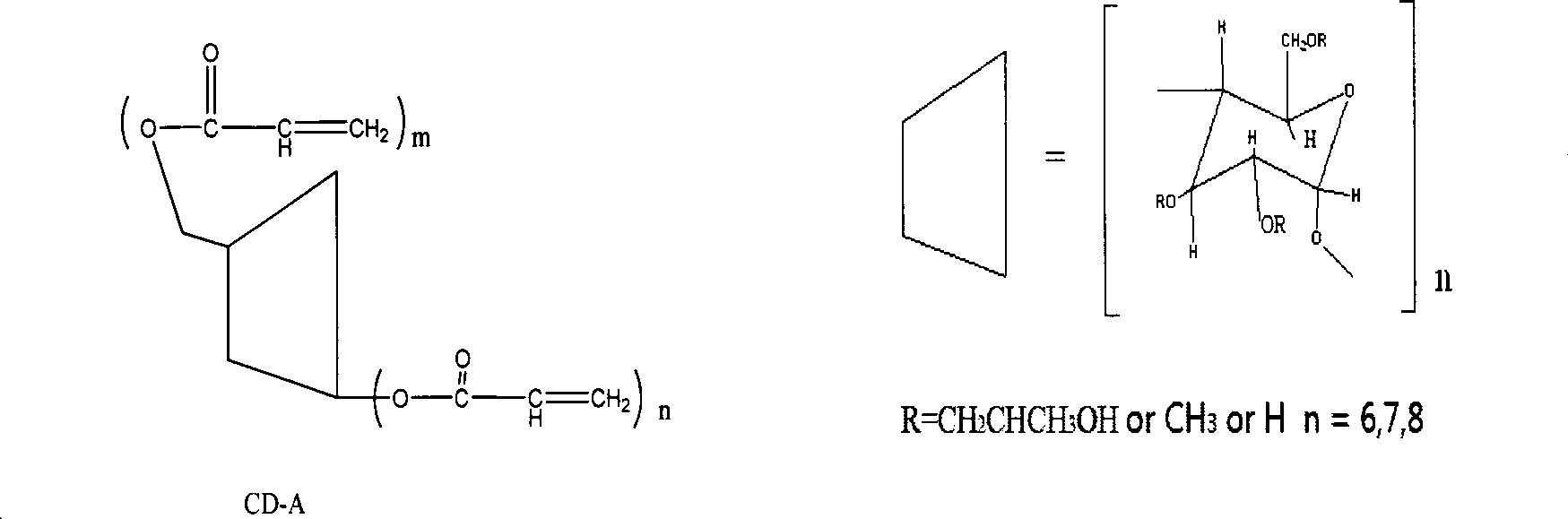
New method for preparing cyclodextrin (meth)acrylate
A technology of cyclodextrin and acrylic acid, which is applied in the field of cyclodextrin acrylate and its preparation, can solve the problems of cumbersome preparation process and unsuitability for industrial production, and achieve broad application prospects, good biocompatibility, and easy to wash off Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Example 1: First, 1.70 g of vacuum-dried β-cyclodextrin was dissolved in 12 ml of anhydrous sodium sulfate-dried dimethylformamide (DMF), dissolved under magnetic stirring, and then 0.1 g of 4-(N, N -Dimethylamino)pyridine (DMAP) dissolved. Under the condition of ice-water bath at 0°C, slowly add 1ml of ice-stored acylating agent (meth)acryloyl chloride dropwise into the reaction solution, after the dropwise addition is completed, slowly warm up to room temperature, and react for 12 hours. After the reaction is complete, add 100ml of acetone to the reaction solution to precipitate, filter, collect the white filter residue, and vacuum dry at 40°C. After 72 hours, a white product was obtained.
example 2
[0025] Example 2: First, 1.70g of vacuum-dried β-cyclodextrin was dissolved in 10ml of dimethylformamide (DMF), dissolved under magnetic stirring, and then 0.1g of 4-(N,N-dimethylamino)pyridine (DMAP) was added ) dissolved. Add 1ml of ice-stored acylating agent (meth)acryloyl chloride to dilute with 2ml of dimethylformamide (DMF), and slowly add it dropwise into the reaction solution with a constant pressure dropping funnel under the condition of 0°C ice-water bath. , slowly rose to room temperature, and reacted for 12h. After the reaction is complete, add 100ml of acetone to the reaction solution to precipitate, filter, collect the white filter residue, and vacuum dry at 40°C. After 72 hours, a white product was obtained.
example 3
[0026] Example 3: First, 3.40g of vacuum-dried β-cyclodextrin was dissolved in 20ml of dimethylformamide (DMF), dissolved under magnetic stirring, and then 0.2g of 4-(N,N-dimethylamino)pyridine (DMAP ) dissolved. After adding 6ml of ice-stored acylating agent (meth)acryloyl chloride to 12ml of dimethylformamide (DMF) to dilute, slowly drop into the reaction solution with a constant pressure dropping funnel under the condition of 0°C ice-water bath, dropwise After completion, it was slowly raised to room temperature and reacted for 12 hours. After the reaction is complete, add 300ml of acetone to the reaction solution to precipitate, filter, collect the white filter residue, and vacuum dry at 40°C. After 72 hours, a white product was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com