Sulfonic acid group-containing polymer, sulfonic acid group-containing aromatic compound and method of making same, as well as polymer electrolyte material, polymer electrolyte molded product and solid polymer fuel cell using same
A technology of aromatic compounds and electrolyte materials, applied in the direction of solid electrolyte fuel cells, sulfonic acid preparation, fuel cell parts, etc., can solve the problems of increased number of reaction steps, increased costs, insufficient chemical stability, etc., and achieve high output , excellent physical durability, the effect of excellent proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0324] Synthesis of 2,2-bis(4-hydroxyphenyl)-1,3-dioxolane (K-DHBP) having the following formula (G1):
[0325] [chemical formula 29]
[0326]
[0327] Add 495 grams of 4,4'-dihydroxybenzophenone, 134 grams of ethylene glycol, 969 grams of trimethyl orthoformate, and 050 grams of p-Toluenesulfonic acid monohydrate, the material was dissolved. The temperature was then maintained at 78 to 82°C and the mixture was stirred for 2 hours. Then, the internal temperature was gradually increased to 120° C., and heating was carried out until the distillation of methyl formate, methanol and trimethyl orthoformate was completely stopped. After cooling to room temperature, the reaction solution was diluted with ethyl acetate, the organic layer was washed with 100 mL of 5% potassium carbonate aqueous solution, the layers were separated, and the solvent was evaporated. 80 ml of dichloromethane was added to the residue to precipitate crystals, which were filtered and dried to obtain 52.0...
Embodiment 1
[0334] (Synthesis of tetrasodium 3,5,3',5'-tetrasulfonic acid-4,4'-difluorodiphenylketone having the following formula (G2))
[0335] Add 1091 grams of 4,4'-difluorodiphenyl ketone to a 1000 ml 3-necked flask equipped with a stirrer and a concentrator
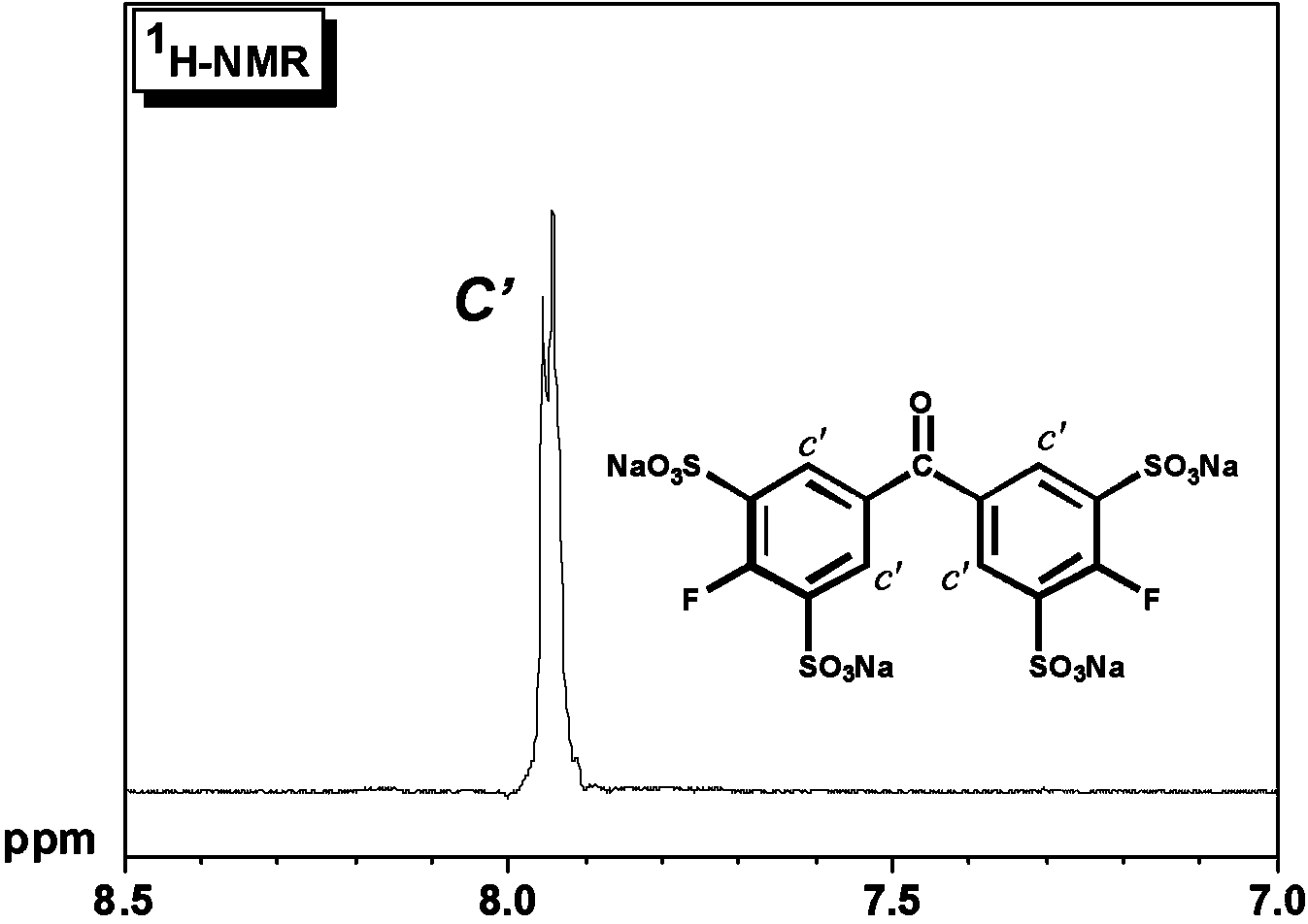
[0336] (Aldrich reagent), 210 milliliters of oleum (60% SO 3 ) (Aldrich reagent), then forcefully pass nitrogen gas into the nitrogen conduit connected to the upper part of the concentrator and the bubbler leading to the outside of the system, and react at 180 ° C for 24 hours. Suppress the evaporation of sulfur trioxide by forcefully blowing nitrogen. The reactant was then slowly placed in a large amount of water, neutralized with NaOH, precipitated with ethanol three times, and sodium sulfate was removed to obtain an aromatic compound containing a sulfonic acid group having the following formula (G2). by 1 H-NMR confirmed the structure. The starting material, disulfonated product, and trisulfonated product were not detect...
Embodiment 2
[0342] (Synthesis of 3,5,3',5'-tetrasulfonic acid-4,4'-dichlorodiphenylsulfone tetrasodium having the following formula (G3))
[0343] Add 143.6 grams of 4,4'-dichlorodiphenyl sulfone in a 1000 ml 3-necked flask equipped with a stirrer and a concentrator
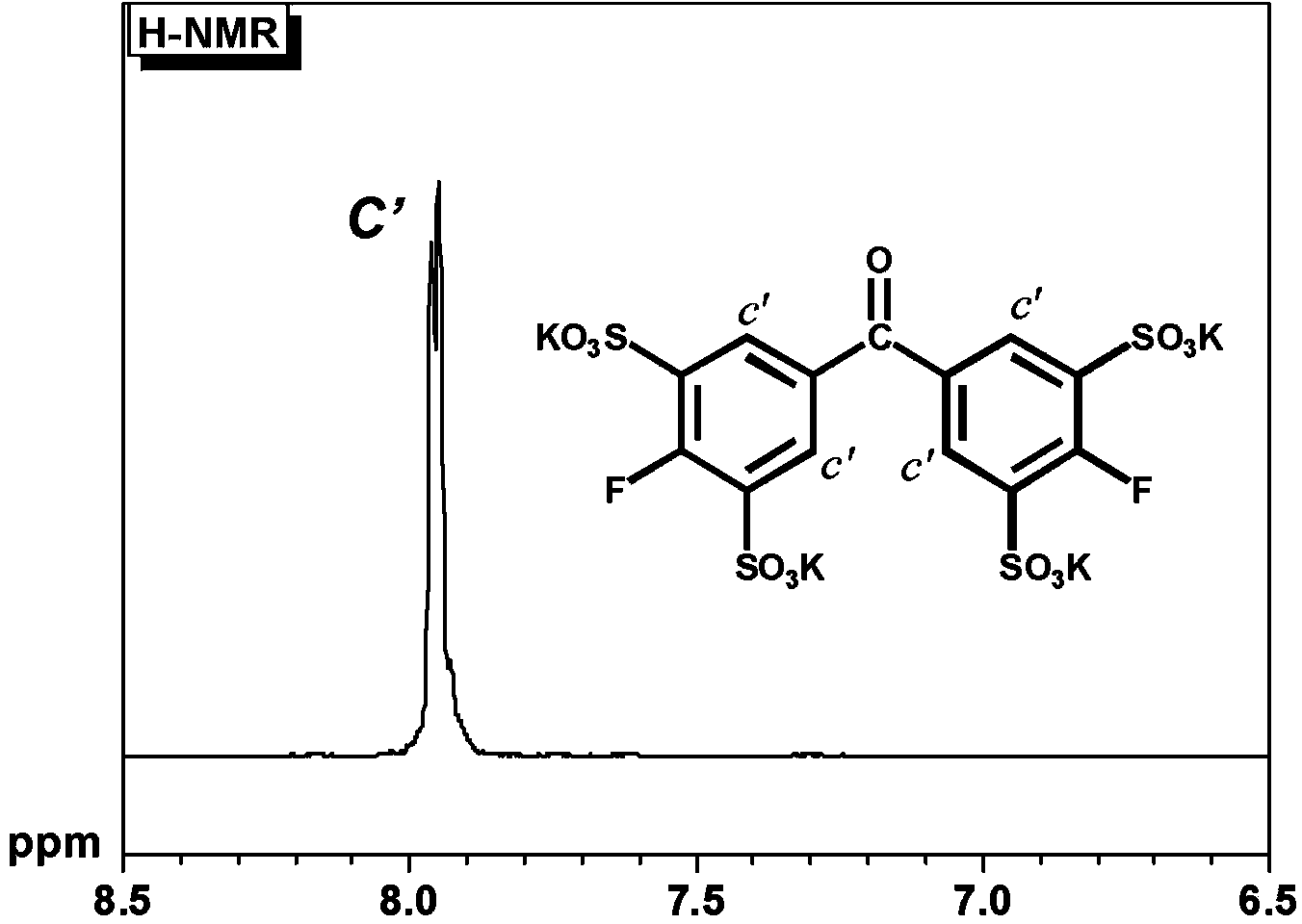
[0344] (Aldrich reagent), 210 milliliters of oleum (60% SO 3 ) (Aldrich reagent), then forcefully pass nitrogen gas into the nitrogen conduit connected to the upper part of the concentrator and the bubbler leading to the outside of the system, and react at 200 ° C for 24 hours. At this time, the vigorously flowing nitrogen suppresses the volatilization of sulfur trioxide. After the reaction solution was gradually placed in a large amount of water and neutralized with NaOH, sodium sulfate was removed by ethanol precipitation three times to obtain a sulfonic acid group-containing aromatic compound having the following formula (G3). by 1 H-NMR confirmed the structure. It did not identify the starting material, disulfonated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| Resonant frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com