Supported precious metal methanation catalyst, and preparation method and application thereof
A technology for methanation catalyst and precious metal oxide, applied in the field of supported precious metal methanation catalyst and preparation, can solve the problems of agglomeration, small pore size, small specific surface area, etc., and achieves reduction of preparation cost, improvement of methanation activity, and improvement of dispersibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
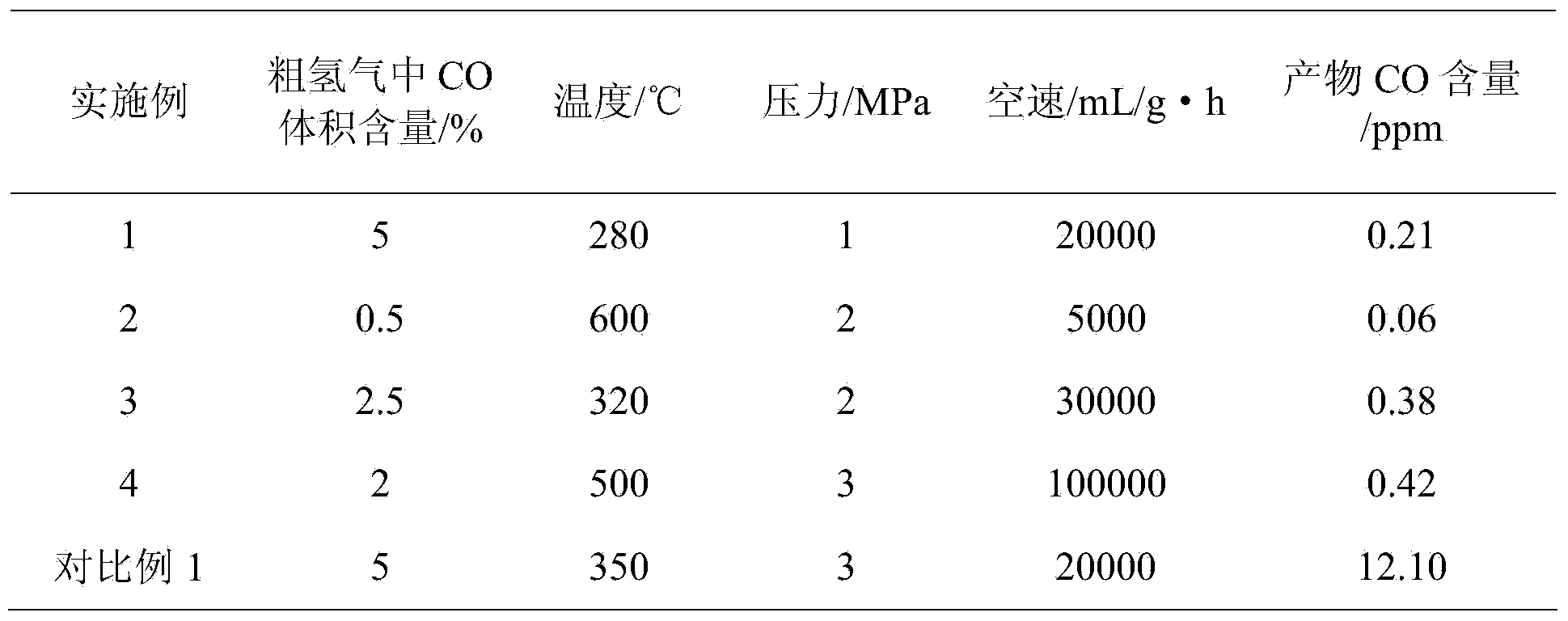
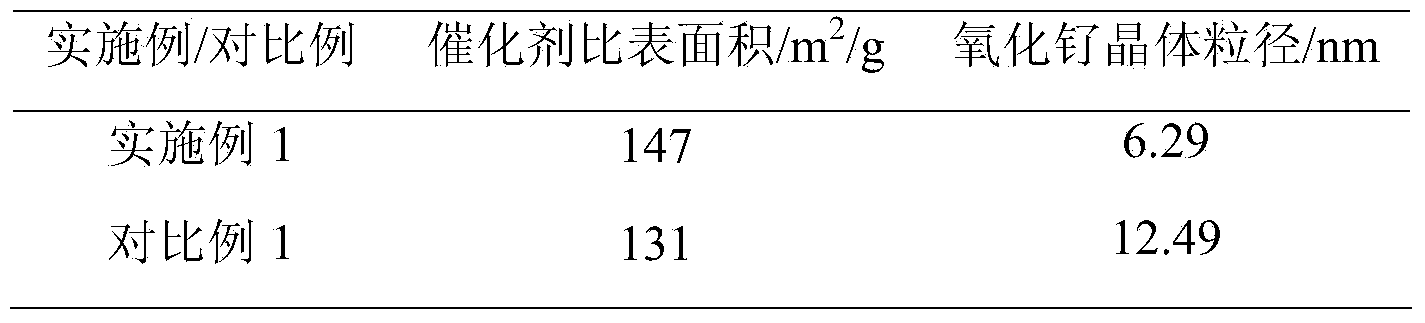
[0023] Weigh 1.2g of ruthenium nitrate and 2g of urea, dissolve in 10mL of water, add 9.5g of alumina, impregnate and stir at room temperature for 24h, pour the suspension into a ceramic evaporating dish, put it in a muffle furnace, and heat to 500°C , self-combustion, the powder after combustion is collected, ground and granulated to 60-100 mesh to obtain a methanation catalyst. The catalyst composition is: RuO 2 5wt.%, Al 2 o 3 95wt.%. Put a certain mass of the above catalyst into a fixed bed reactor, at a temperature of 450°C, the gas volume composition is 15%H 2 with 85%N 2 , reduced for 6h at a space velocity of 3000mL / (g·h). The reduced catalyst is used for fixed-bed methanation reaction, and the specific reaction conditions and results are shown in Attached Table 1.
Embodiment 2
[0025] Weigh 0.5g rhodium nitrate and 5g ethylene glycol, dissolve in 10mL water, add 9.8g zirconium dioxide, impregnate and stir at room temperature for 24h, pour the suspension into a ceramic evaporating dish, put it in a muffle furnace, and heat At 400°C, it burns by itself, collects the remaining powder after combustion, grinds it and granulates it to 100-140 mesh to obtain a methanation catalyst. The catalyst consists of: Rh 2 o 3 2wt.%, ZrO 2 98wt.%. Put a certain mass of the above-mentioned catalyst into a fixed-bed reactor, at a temperature of 650 °C, and a gas volume composition of 10% H 2 with 90%N 2 , reduced for 2h at a space velocity of 5000mL / (g·h). The reduced catalyst is used for fixed-bed methanation reaction, and the specific reaction conditions and results are shown in Attached Table 1.
Embodiment 3
[0027] Weigh 0.6g of palladium nitrate and 2g of glycine, dissolve in 10mL of water, add 9.7g of cerium oxide, impregnate and stir at room temperature for 24h, pour the suspension into a ceramic evaporating dish, put it into a muffle furnace, and heat to 300 ℃, self-combustion, the remaining powder after combustion is collected, ground and granulated to 120-160 mesh to obtain a methanation catalyst. The catalyst composition is: PdO3wt.%, CeO 2 97wt.%. Put a certain mass of the above-mentioned catalyst into a fixed-bed reactor, at a temperature of 400 °C, and a gas volume composition of 5% H 2 with 95%N 2 , reduced for 5h at a space velocity of 10000mL / (g·h). The reduced catalyst is used for fixed-bed methanation reaction, and the specific reaction conditions and results are shown in Attached Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com