Fluoroquinolone medicine composition
A fluoroquinolone and composition technology, applied in the field of veterinary fluoroquinolone pharmaceutical compositions, can solve the problems of difficult preparation, inconvenience and difficulty in use, etc., and achieve the effects of simple and easy operation of the preparation method, increased therapeutic effect, and improved drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0017] The enrofloxacin used in the present invention was purchased from Zhejiang Guobang Pharmaceutical Co., Ltd., with a content of 98.5%, and batch number 120222-1; the enrofloxacin hexahydrate was prepared by Luoyang Huizhong Veterinary Medicine Co., Ltd. according to CN101961335A Example 1, with a content of 75.0%, batch number 20120102; Ciprofloxacin was purchased from Zhejiang Guobang Pharmaceutical Co., Ltd., with a content of 96.7%, batch number 111020-21.
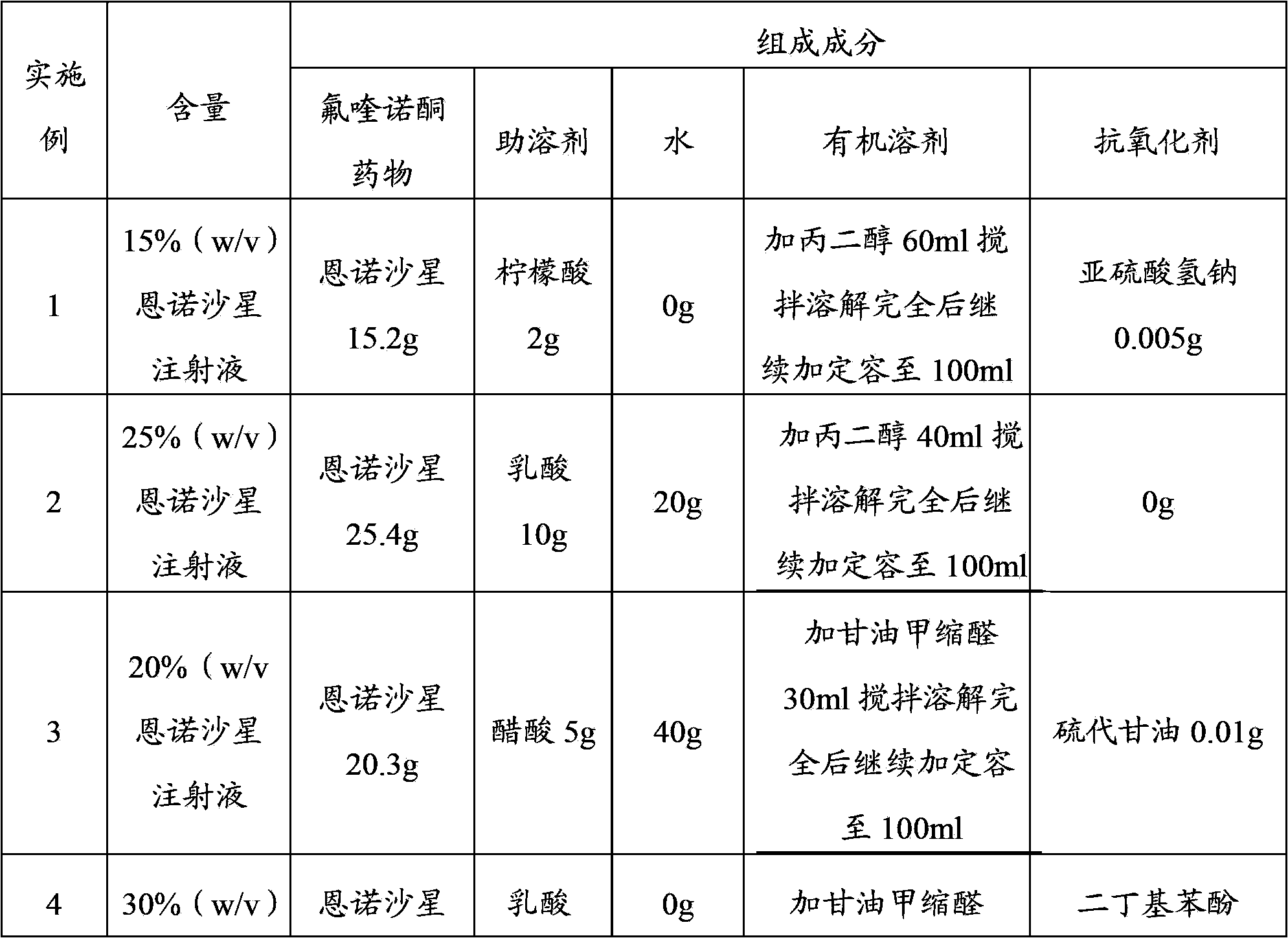
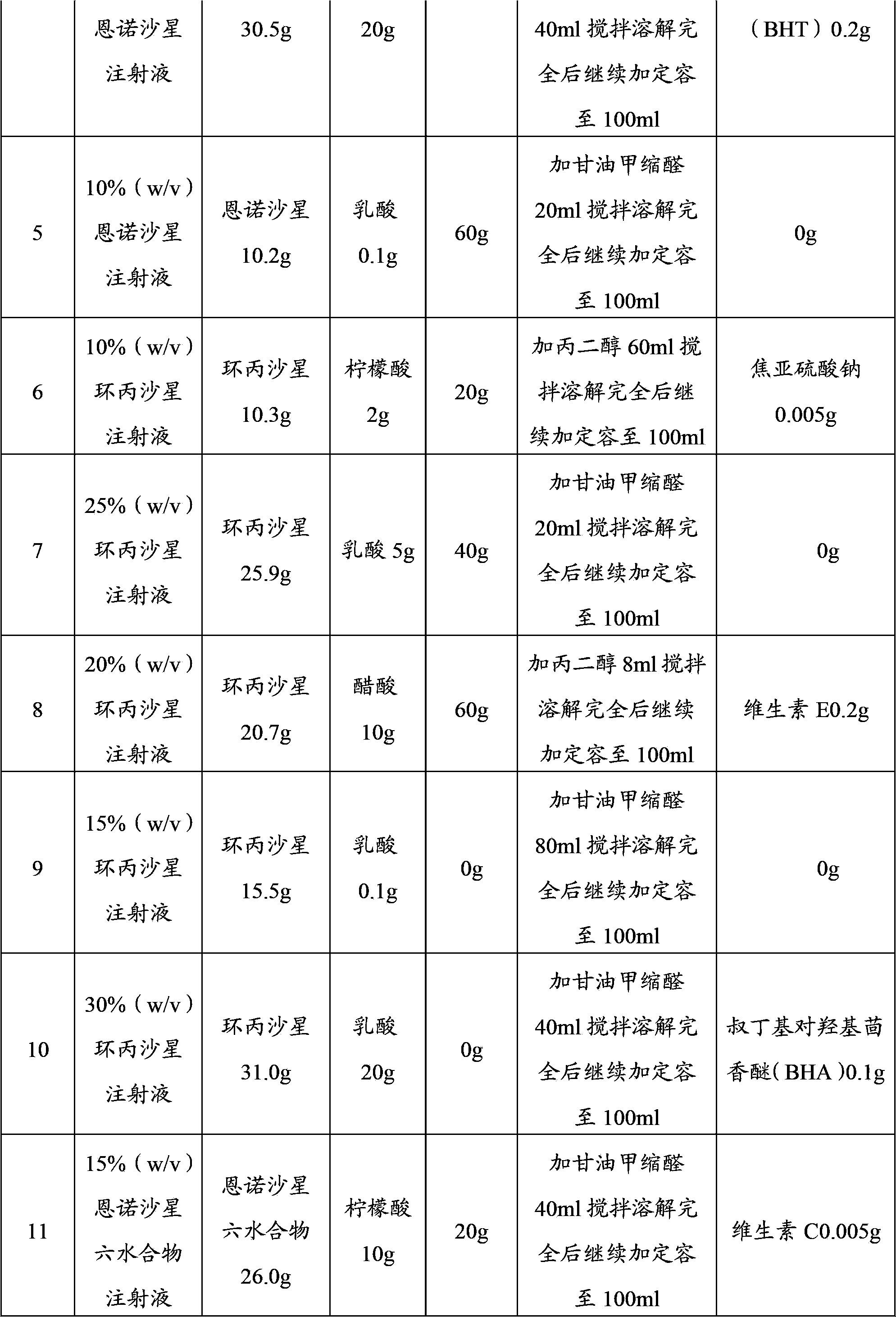
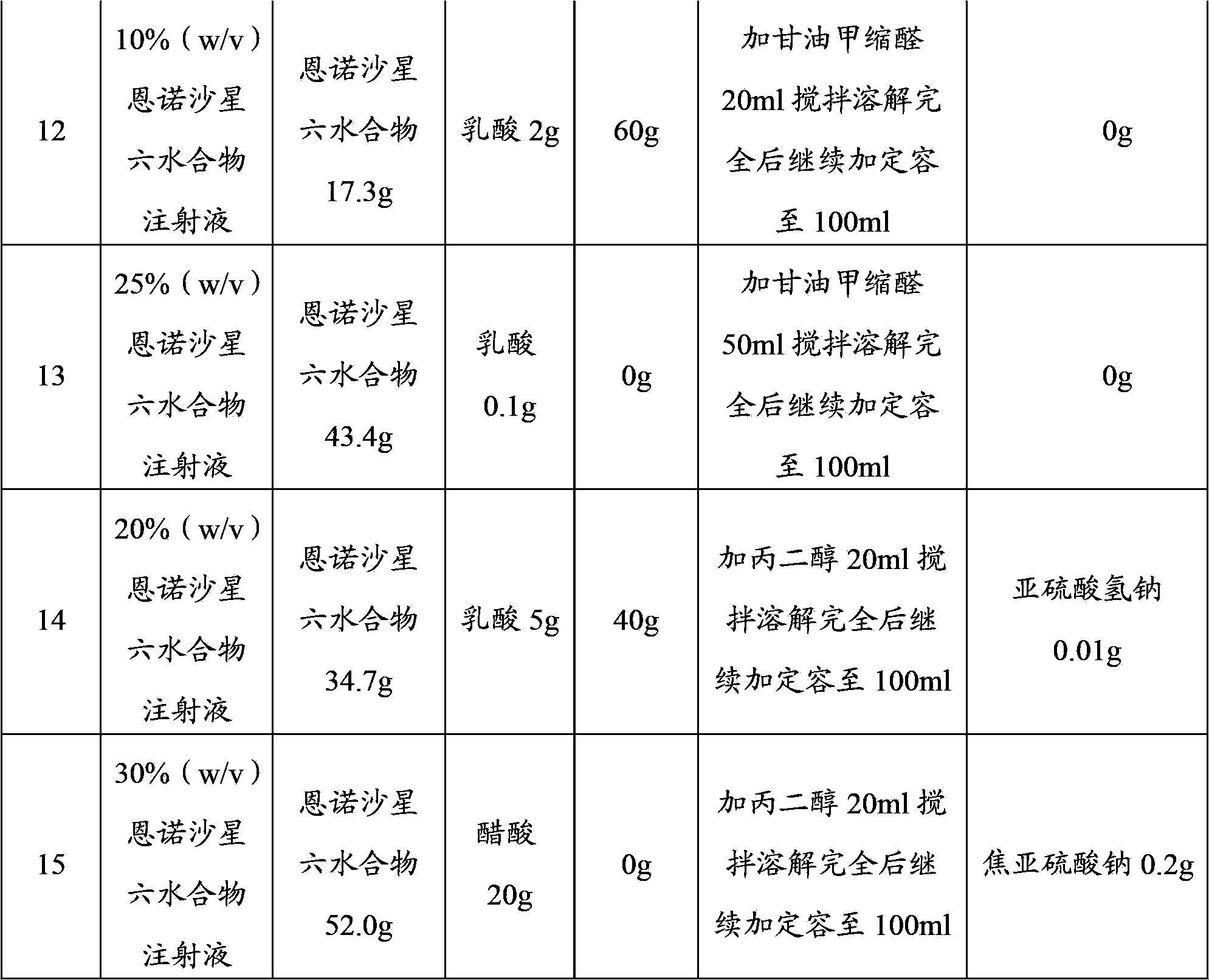
[0018] Examples 1-15 prepared according to the technical solution of the present invention are shown in Table 1:
[0019] Table 1
[0020]
[0021]
[0022]
[0023] Note: The content of enrofloxacin hexahydrate is calculated by enrofloxacin.
[0024] Preparation:
[0025] Add the prescribed amount of enrofloxacin (or enrofloxacin hexahydrate, ciprofloxacin), co-solvent, water and antioxidants to an appropriate amount of organic solvent according to the above prescriptions, stir until completely disso...
Embodiment 16
[0026] Embodiment 16 comparative example composition
[0027] According to the Chinese patent CN101810569A, Example 1 discloses a preparation method of enrofloxacin injection comprising the following steps:
[0028] (1) Add 20 grams of enrofloxacin into 60ml of aqueous sodium hydroxide solution with a concentration of 0.4mol / l, and stir until the enrofloxacin is completely dissolved;
[0029] (2) Continue to add sodium hydroxide aqueous solution with a concentration of 0.4mol / l to adjust the pH value to 12;
[0030] (3) Add 20ml of acidulant lactic acid. During the addition of acidulant, enrofloxacin will first crystallize. With the addition of acidulant, when the pH value reaches neutral, enrofloxacin will completely dissolve;
[0031] (4) Add 0.5g sodium thiosulfate antioxidant to the neutral solution;
[0032] (5) Continue to add 0.5g of EDTA chelating agent;
[0033] (6) After step (5) is completed, dilute to 100ml with purified water, and filter the dilute solution thr...
Embodiment 17、15
[0036] Embodiment 17, 15% enrofloxacin injection
[0037] Enrofloxacin injection, each 100ml injection consists of the following ingredients: 15.2g enrofloxacin, an appropriate amount of 4% (w / v) sodium hydroxide aqueous solution, add water to 100ml.
[0038] Preparation method: Weigh 15.2g of enrofloxacin, suspend it in 50ml of water, add dropwise an appropriate amount of 4% (w / v) sodium hydroxide aqueous solution while stirring, adjust the pH value to 10, and dilute the water to 100ml.
[0039] The 15% enrofloxacin injection prepared in this example was placed at room temperature for 3 hours, and crystals were precipitated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com