Polyelectrolyte-containing drug coating and preparation method thereof
A drug coating, polyelectrolyte technology, applied in the field of medical devices, can solve problems such as uncontrollable and slow drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
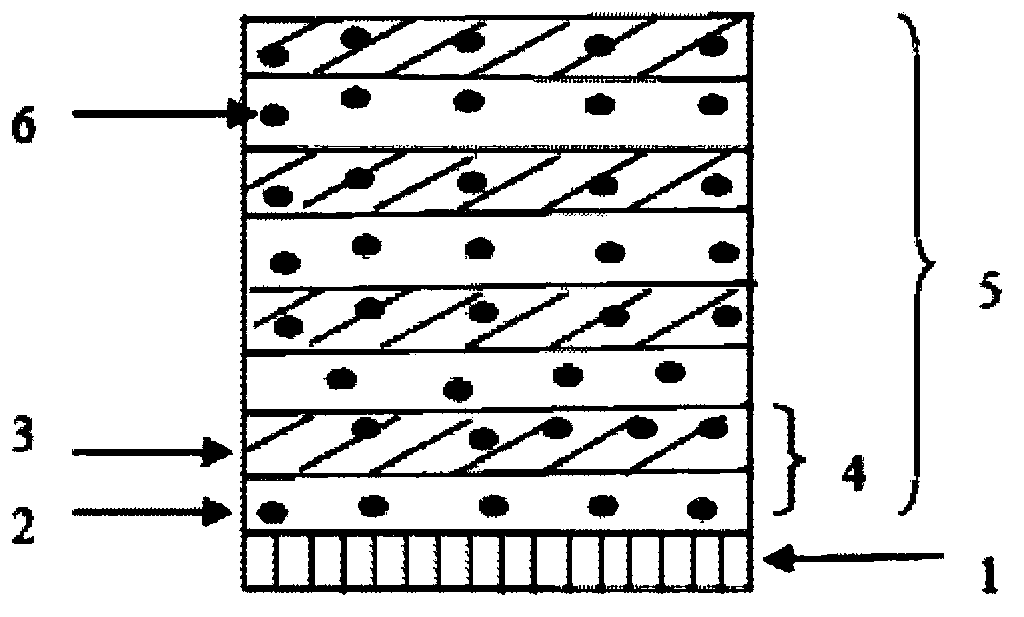
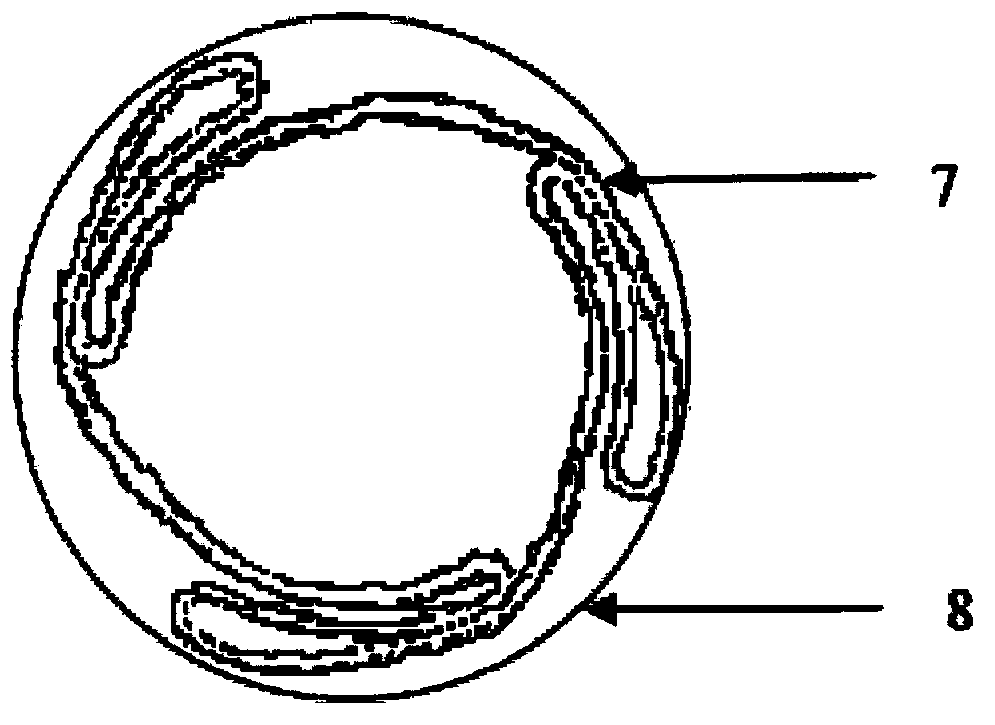
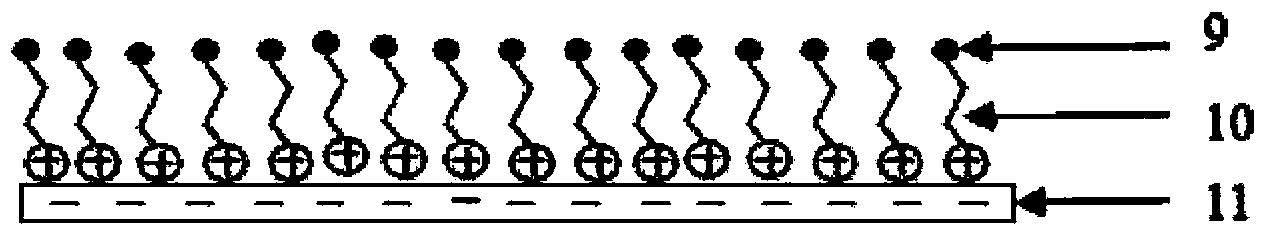
[0028] Prepare polyethyleneimine aqueous solution with a concentration of 0.2g / ml (solution A), 0.2g / ml type A gelatin NaOH aqueous solution (pH=9, solution B), and paclitaxel dichloromethane solution with a concentration of 50mg / ml ( Solution C), 30% PVP (molecular weight 10,000) in isopropanol IPA solution (solution D). Immerse the balloon in solution A for 1min, after drying, immerse it in solution C for 10min, and dry it, then immerse it in solution B for 1min, and rinse it with double distilled water for 3 times, after drying, immerse it in solution C for 10min, repeat the above coating operation 5 times, fold the coated balloon and dip it into 30% PVP (molecular weight: 10,000) isopropanol IPA solution, then dry and sterilize with ethylene oxide to obtain the drug balloon.
Embodiment 2
[0030] Prepare chitosan solution with a concentration of 0.2g / ml (solution A), 0.2g / ml type A gelatin NaOH aqueous solution (pH=9, solution B), and paclitaxel ethanol solution with a concentration of 50mg / ml (solution C) , 30% PVP (molecular weight 10,000) in isopropanol IPA solution (solution D). Immerse the balloon in solution A for 1min, after drying, immerse it in solution C for 10min, and dry it, then immerse it in solution B for 1min, and rinse it with double distilled water for 3 times, after drying, immerse it in solution C for 10min, repeat the above coating operation 5 times, fold the coated balloon and dip it into 30% PVP (molecular weight: 10,000) isopropanol IPA solution, then dry and sterilize with ethylene oxide to obtain the drug balloon.
Embodiment 3
[0032] In vitro release test
[0033] Take a section of 6F catheter and immerse it in pH 7.4 phosphate buffer solution, keep it at a constant temperature of 37°C, pass the drug balloon through the catheter in the folded state and keep it for 1 min, take out the drug balloon, measure the drug residue by HPLC, and calculate the drug during delivery loss rate.
[0034] Take another balloon, repeat the above operation, expand the balloon, keep the expanded state for 2 minutes, take out the drug balloon, measure the drug residue by HPLC, and calculate the drug release during the expansion process.
[0035] tissue absorption test
[0036] Take an isolated porcine arterial segment that is about 20% larger than the diameter of the balloon, and immediately immerse it in pig blood added with anticoagulant, keep a constant temperature of 37°C, pass the drug balloon through the 6F catheter in the folded state and keep it for 1min, and then the balloon enters The lumen of the blood vesse...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com