Method for preparing 1, 2-propanediol by adopting jerusalem artichoke as raw material in direct catalytic conversion
A catalytic conversion, propylene glycol technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of poor catalyst stability, difficulty in industrialization, unstable glycerin source, etc., to achieve easy separation and reduce production cost, the effect of contributing to industrial utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Catalyst preparation:
[0020] Activated carbon (AC) used in the present invention all adopts mass concentration 20-50% nitric acid 80 ℃ of soaking 24 hours to carry out pretreatment.
[0021] (1) Preparation of isomerization catalysts: unsupported catalysts such as tin tetrachloride are commercial products purchased directly. In the supported catalyst, the active component is loaded on the carrier, and the carrier is a composite carrier of one or more of activated carbon, alumina, silicon oxide, silicon carbide, zirconia, zinc oxide, and titanium dioxide, and chromium, tin, The rhenium metal salt aqueous solution was respectively loaded on the carrier by equal volume impregnation method, then stood for 2 h, dried at 120°C overnight, and then reduced under hydrogen atmosphere.
[0022] Before the above supported catalysts are used, they need to be reduced with hydrogen at a certain temperature for 1h, and in 1%O 2 / N 2 Passivation under (V / V) atmosphere for 4h.
[0...
Embodiment 2
[0029] Catalytic conversion experiment: the reaction was carried out in a batch reactor, 0.5g Jerusalem artichoke, 0.05g isomerization catalyst, 0.3g hydrogenation catalyst, 0.3g C-C bond breaking catalyst and 50ml water were added to a 100ml reactor, and then passed After entering the hydrogen to replace the gas three times, the hydrogen was charged to 6MPa, the temperature was raised to 245°C, and the reaction was carried out for 80min.
[0030] Alternatively, the reaction is carried out in a semi-continuous reactor, 0.5g of Jerusalem artichoke is dissolved in water, and the insoluble matter is filtered off to form 25ml of Jerusalem artichoke aqueous solution, and 0.05g of isomerization catalyst, 0.15g of hydrogenation catalyst, and 0.15g of C-C bond are broken Catalyst and 25ml water join in the 100ml reactor, when reactor temperature was raised to 245 ℃, with the pump speed of 0.5ml / min, 25ml Jerusalem artichoke solution was injected in reactor by pump, reacted 30min.
[0...
Embodiment 3
[0033] Under the action of a small amount of isomerization catalyst and the C-C bond breaking bond catalyst, the influence of different hydrogenation catalysts on the catalytic conversion of Jerusalem artichoke to prepare 1,2-propanediol is shown in Table 1. The feeding method and reaction conditions are the same as in Example 2 in batch A description of the reaction carried out in the reactor.
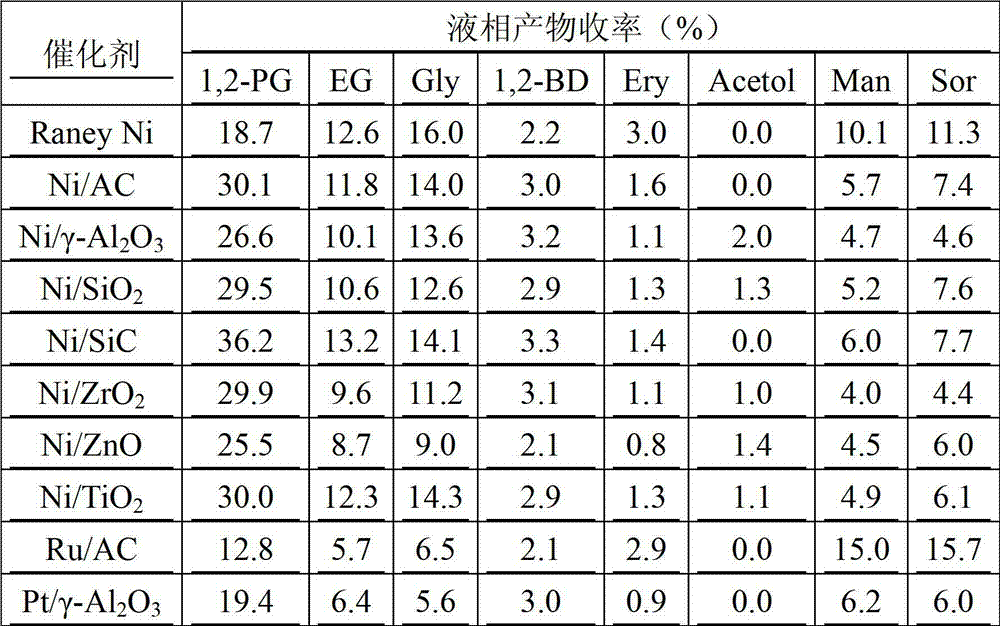
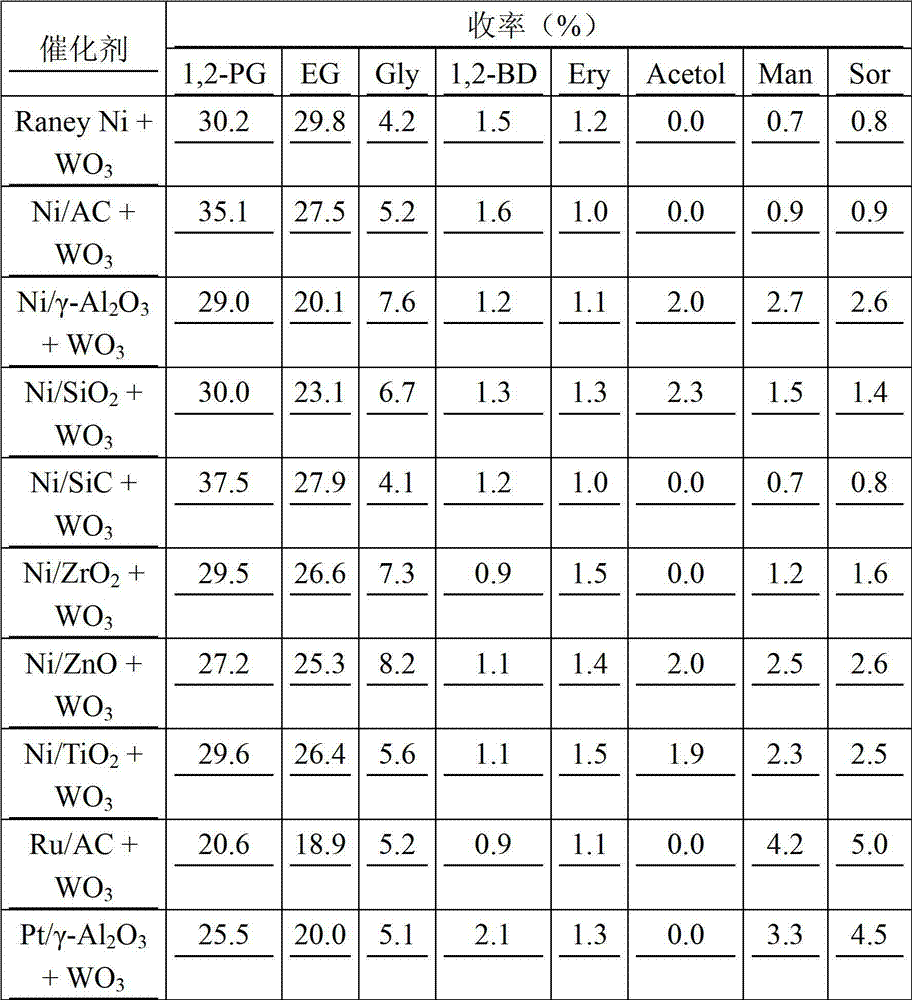
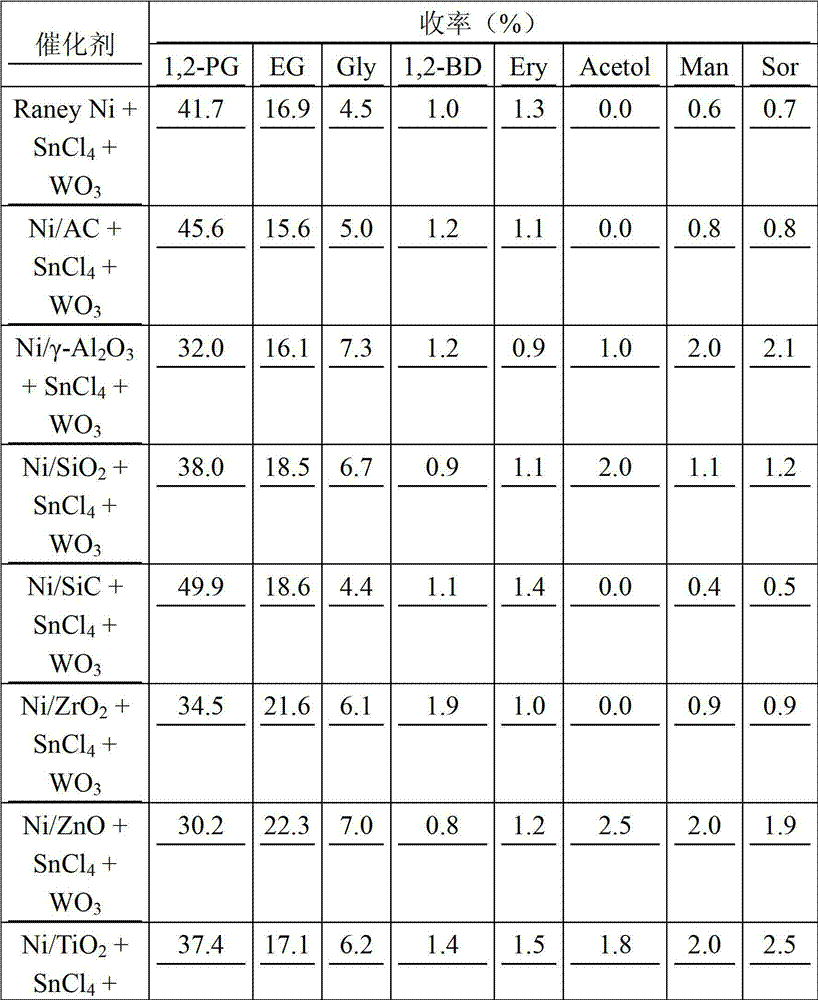
[0034] Table 1 The reaction results of the catalytic conversion of Jerusalem artichoke to prepare 1,2-propanediol under the action of different hydrogenation catalysts (SnCl 4 0.01g, WO 3 0.01g, hydrogenation catalyst 0.3g, Jerusalem artichoke mass concentration 1%)
[0035]
[0036] As shown in Table 1, under the action of a certain amount of isomerization catalyst and C-C bond breaking catalyst, the yield of 1,2-propanediol and ethylene glycol produced from Jerusalem artichoke is relatively low when the intermittent feeding method of Jerusalem artichoke is adopted. Low, the yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com