Chemical sensor for dopamine detection, chemical sensor preparation method, dopamine detection method and application of chemical sensor
A sensor and dopamine technology, applied in the field of chemical sensors, can solve the problems of high equipment requirements, poor selectivity, and low sensitivity, and achieve the effects of high detection sensitivity, easy operation, and obvious selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This example is used to illustrate the preparation of gold-containing nanoparticles.
[0061] All glassware is pre-washed and oven-dried before use using aqua regia and deionized water. In a 250ml round bottom flask, heat 100ml of 1.0mM tetrachloroauric acid (HAuCl 4 4H 2 O) When the aqueous solution boils, add 10ml of 38.8mM sodium citrate solution, and continue heating and boiling for 30 minutes. The solution color changed from light yellow to wine red during this time, indicating the formation of gold nanoparticles. Then the solution was cooled, centrifuged and washed with deionized water several times, and filtered with a filter membrane with a pore size of 0.2 μm to obtain a gold nanoparticle sol.
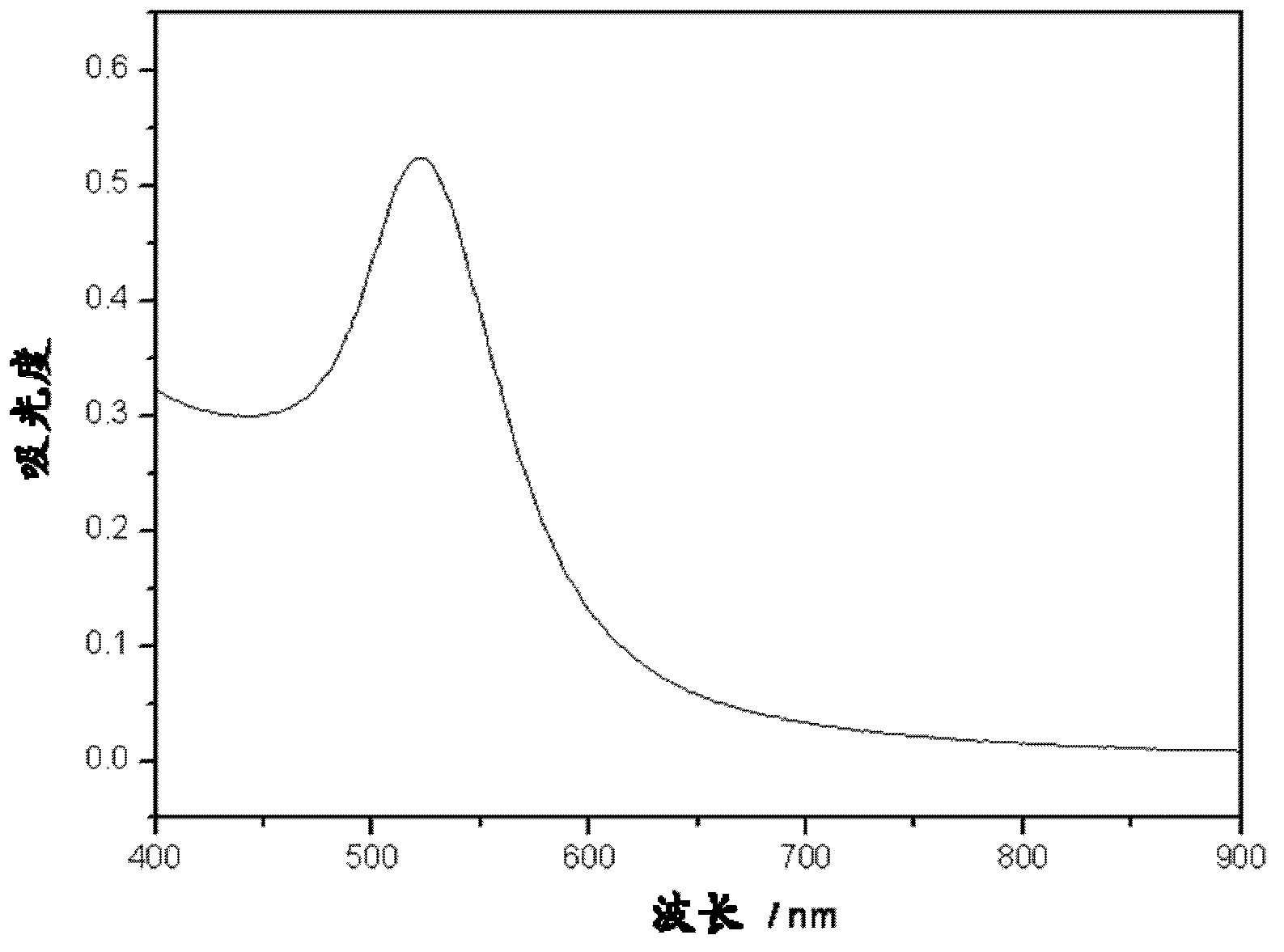
[0062] Determination of the absorption spectrum of the prepared gold nanoparticle sol, such as figure 1 As shown, the calculated concentration of gold nanoparticles is about 11.5nM. The gold nanoparticle sol has the maximum absorption at the wavelength of 521nm, an...
Embodiment 2
[0064] This example is used to illustrate the chemical sensor of the present invention and its preparation.
[0065] Add 2ml of 1mM pyridine-4-boronic acid (PDBA) and 0.5mM of 3,3'-dithiodipropionic acid bis(N-hydroxybutanedi Imide ester) (DSP) solution, stirred for about 2 hours to prepare a chemical sensor, as shown in the following formula:
[0066]
[0067] A total of 20 dopamine solutions were prepared with concentrations of 2 μM, 8 μM, 12 μM, 16 μM, 20 μM, 24 μM, 28 μM, 32 μM, 36 μM, 40 μM, 44 μM, 48 μM, 52 μM, 56 μM, 60 μM, 64 μM, 68 μM, 72 μM, 76 μM, and 80 μM, And mix 5 μl of dopamine solution of different concentrations with 3ml of chemical sensor, the dopamine concentration range after the solution is mixed is 3.3nM~133.3nM, so that different color changes occur in the chemical sensor, specifically as image 3 shown.
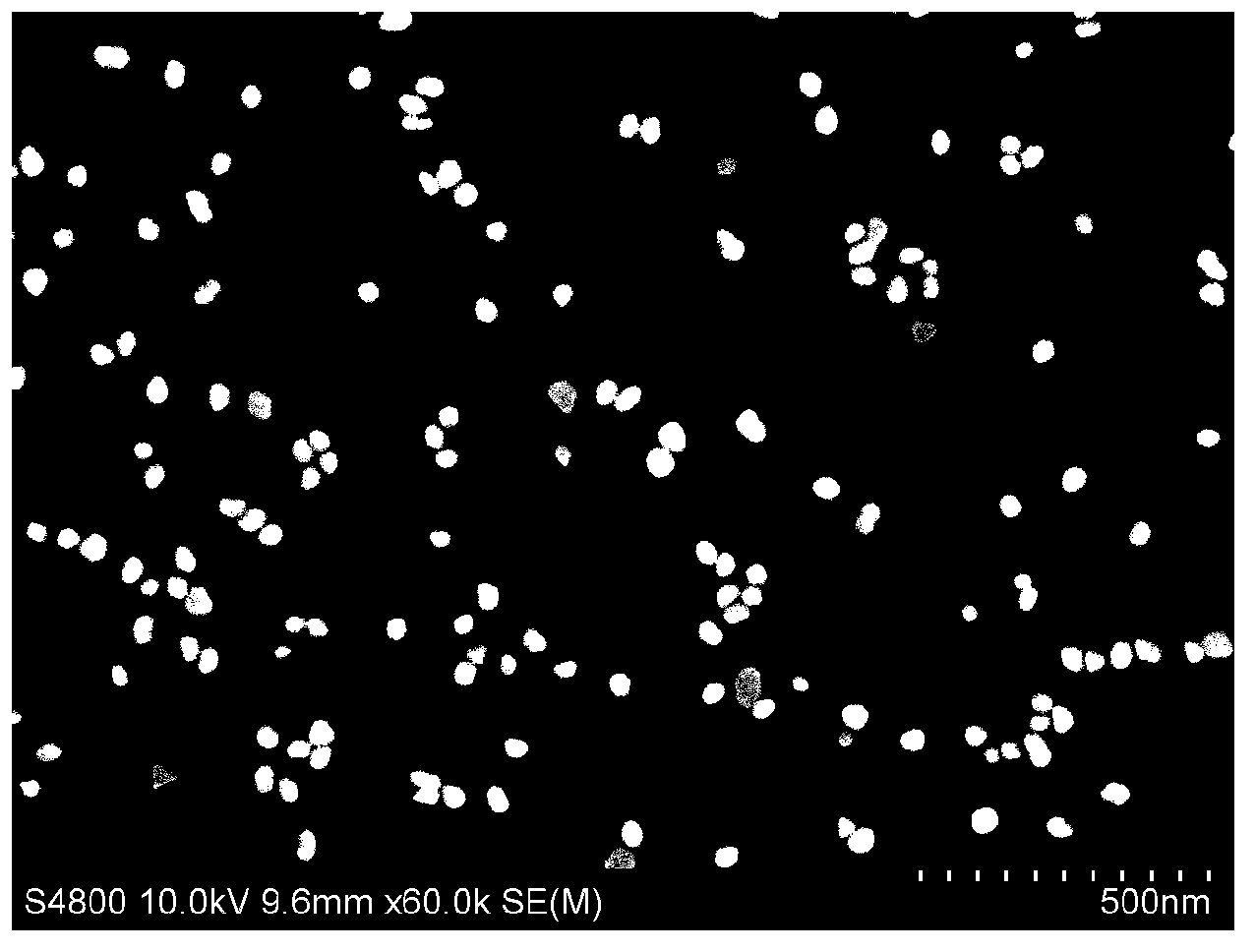
[0068] The aggregation of gold nanoparticles under the concentration of 33.3nM and 100nM dopamine was observed by SEM as follows: Figure 4 wit...
Embodiment 3
[0073] This example is used to illustrate the chemical sensor of the present invention and its preparation.
[0074] Add 2ml of 1mM PDBA and 0.01mM solution of 4-mercaptobenzo18-crown-6 (ABCE) to the 100ml gold nanoparticle sol prepared in Example 1 at room temperature, and stir for about 2 hours to obtain chemical sensor, as follows:
[0075]
[0076] According to the same method as Example 2, prepare different concentrations of dopamine aqueous solution, add 5 μ l of different concentrations of dopamine aqueous solution to the chemical sensor of 3ml, so that the dopamine concentration is in a linear gradient within 3.3 ~ 133.3nM, in the process can be The color of the chemosensors was observed to change (essentially from wine red to dark blue) with the addition of dopamine, and the color varied according to the amount of dopamine added, as shown in Figure 9 As shown, it shows that the gold nanoparticles in the chemical sensor have aggregated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com