Method of preparing conjugated linoleic acid glyceride by utilizing immobilized lipase
A conjugated linoleic acid glyceride, conjugated linoleic acid glyceride technology, applied in the field of preparing conjugated linoleic acid glyceride, can solve the problems of long time, reduce product quality, easy residues, etc., to save cumbersome processes , The effect of reducing the reaction time and reducing the impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
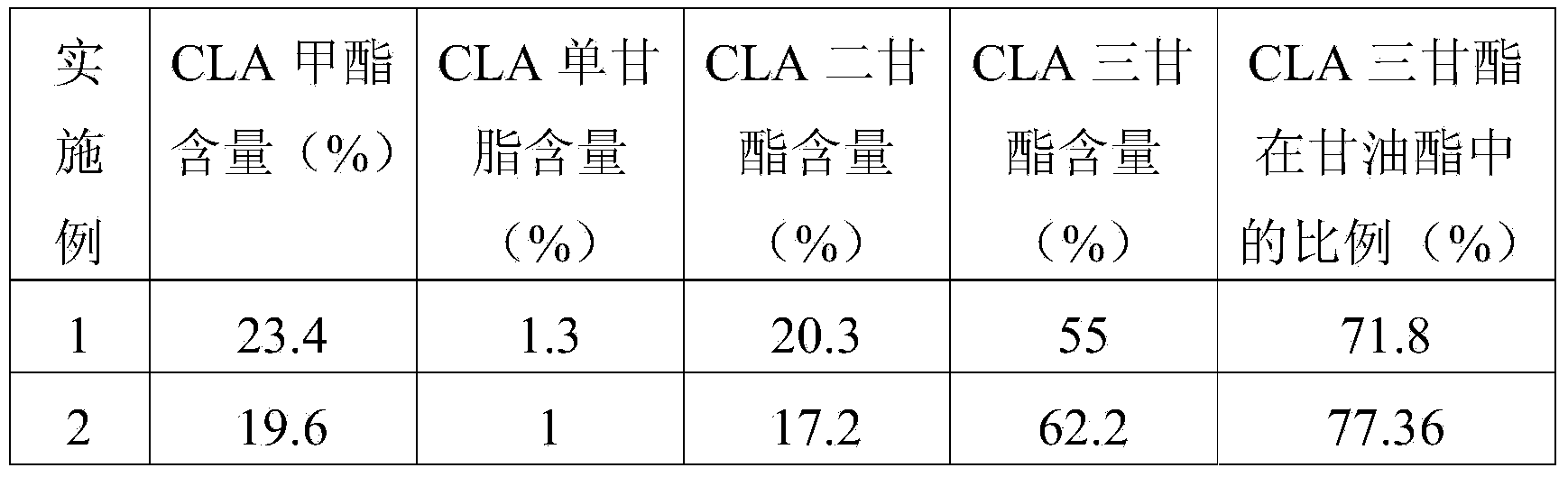
Embodiment 1
[0059] (1) Fill 2.4g of immobilized lipase in the enzyme reaction column, and keep the temperature of the enzyme reaction column at 45°C;
[0060](2) Put methyl conjugated linoleate containing 1% free fatty acid and glycerol in a molar ratio of 3:1 into the material tank, and preheat to 65°C;
[0061] (3) Stir and mix the materials in step (2) evenly at a stirring speed of 100rpm / min. The uniformly mixed material liquid is pumped into the enzyme reaction column through a constant flow pump at a flow rate of 0.5ml / min. The reaction was continued for 35 hours, and the reaction product was collected. Molecular distillation was carried out at 175° C. and 20 Pa to remove excess methyl conjugated linoleate to obtain the final product.
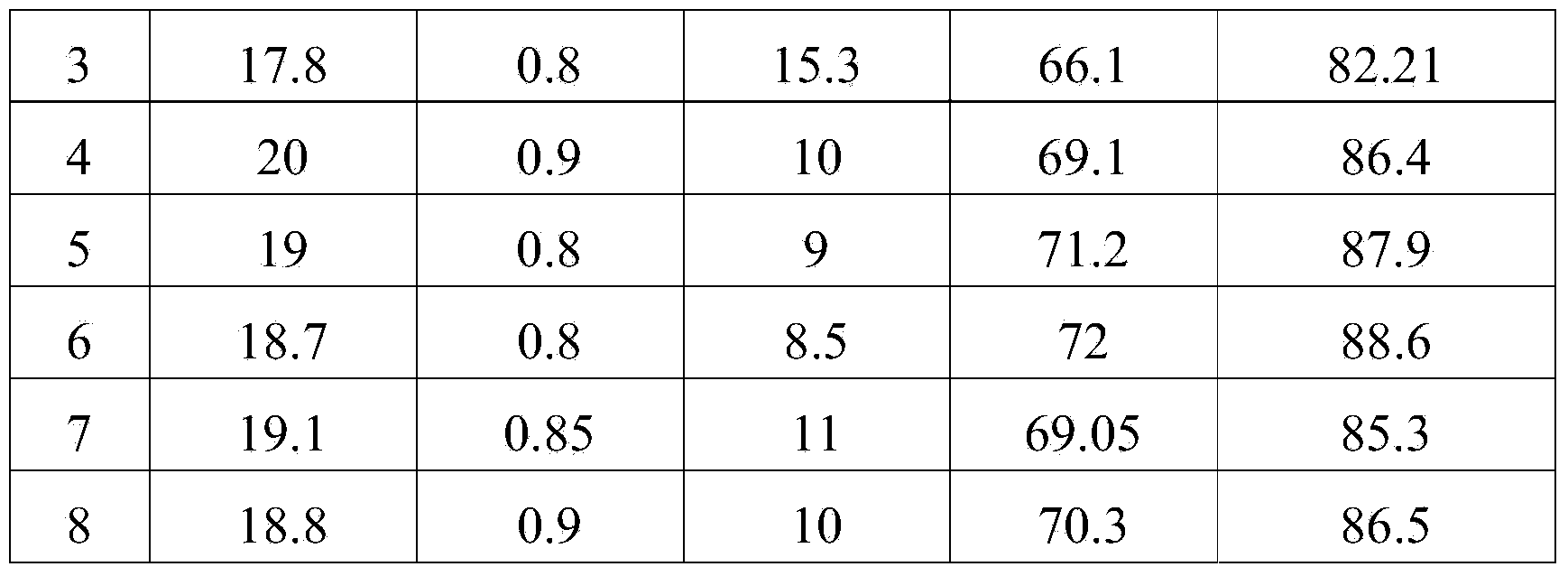
[0062] The composition and content of the final product were determined by high performance liquid chromatography, and the results are shown in Table 1.
Embodiment 2
[0064] (1) Fill 2.4g of immobilized lipase in the enzyme reaction column, and keep the temperature of the enzyme reaction column at 45°C;
[0065] (2) Put the conjugated linoleic acid methyl ester and glycerin with 0% free fatty acid in a molar ratio of 3:1 into the material tank, let it stand, the reaction liquid is divided into two layers, and the upper layer is conjugated linoleic acid methyl ester , the lower layer is glycerol, and the reaction solution is preheated to 65°C;
[0066] (3) Pass the sample tube connected to the constant flow pump into the bottom end of the conjugated methyl linoleate material in the upper layer of the reaction solution, and pump it into the enzyme reaction column through the constant flow pump at a flow rate of 0.5ml / min. After the second time, feed the feed pipe to the bottom of the glycerin layer in the lower layer of the reaction solution, pump it into the enzyme reaction column through a constant flow pump, and the flow rate is 0.5ml / min,...
Embodiment 3
[0070] (1) Fill 2.4g of immobilized lipase in the enzyme reaction column, and keep the temperature of the enzyme reaction column at 50°C;
[0071] (2) Put the conjugated linoleic acid methyl ester containing 5% free fatty acid and glycerol in a molar ratio of 3.5:1 into the material tank, let it stand, the reaction liquid is divided into two layers, and the upper layer is conjugated linoleic acid methyl ester , the lower layer is glycerol, and the reaction solution is preheated to 65°C;
[0072] (3) Pass the sample tube connected to the constant flow pump into the bottom end of the conjugated methyl linoleate material in the upper layer of the reaction solution, and pump it into the enzyme reaction column through the constant flow pump at a flow rate of 0.5ml / min. After the second time, feed the feed pipe to the bottom of the glycerin layer in the lower layer of the reaction solution, pump it into the enzyme reaction column through a constant flow pump, and the flow rate is 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com