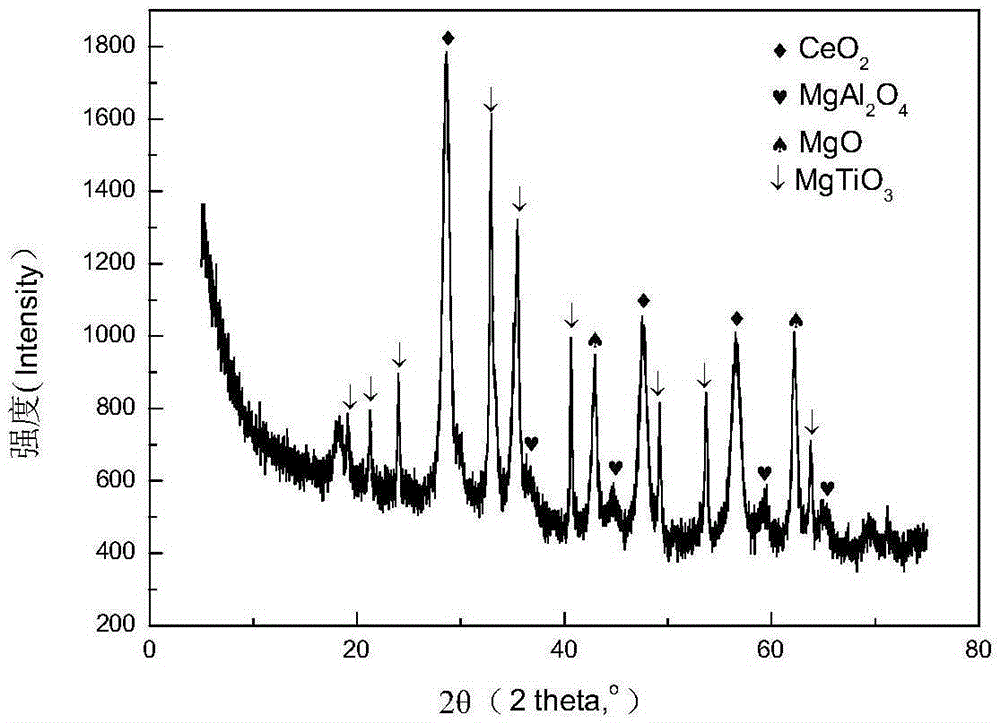
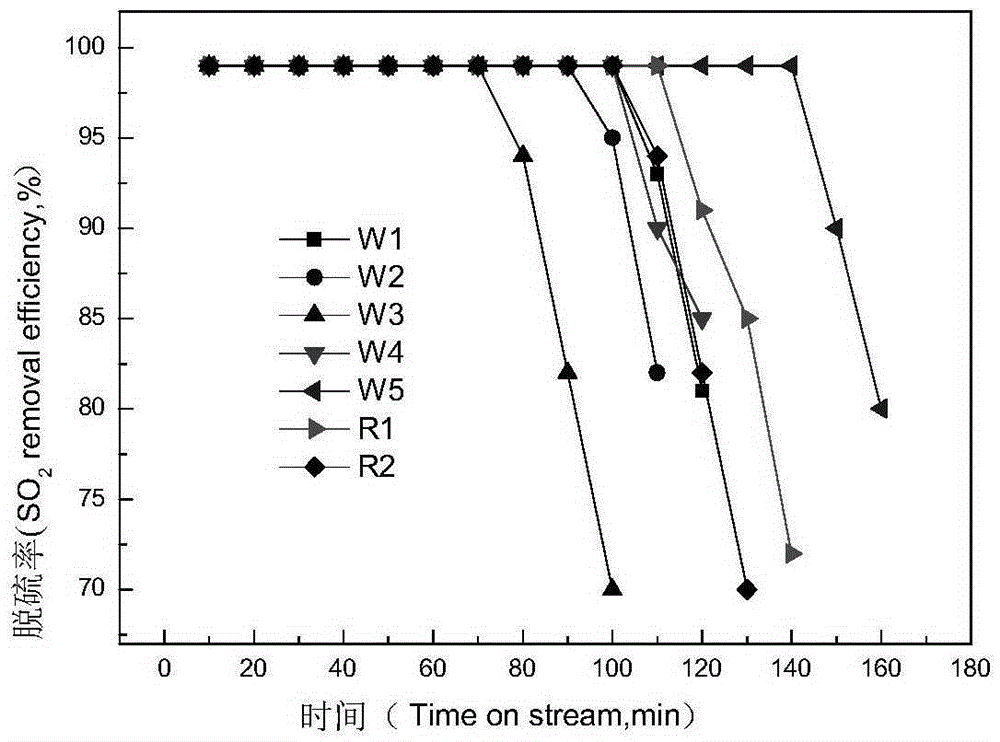
Mixed crystal phase catalytic cracking flue gas sulfur transfer agent and its preparation method and application
A catalytic cracking and sulfur transfer agent technology, applied in chemical instruments and methods, separation methods, dispersed particle separation, etc., can solve problems such as the decrease in the function of silica to enhance strength, reduce the number of sulfur transfer agent desulfurization active centers, etc. Enhance the desulfurization effect and service life, widen the composition range, and improve the desulfurization activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 43.77gMgSO 4 , 16.8gAlCl 3 , 30.16gTi(SO 4 ) 2 9H 2 O, 15.15gFe(NO 3 ) 3 9H 2 O, 9.06gCu(NO 3 ) 2 ·3H 2 O dissolved in 200gH 2 O, prepared as solution A, and stirred at room temperature for 4h; dissolved 30g NaOH in 200gH 2 In O, configure solution B; add solution A dropwise to solution B heated in a 60°C water bath with vigorous stirring (to make the slurry rotate quickly, but not to prevent the slurry from flying out of the beaker), and continue stirring for 4 hours after the addition is completed , to obtain slurry C, keep the pH value of the slurry ≥ 10 during the whole mixing process, put slurry C into a reaction kettle with polytetrafluoroethylene lining, crystallize at 80°C for 18h, then cool, filter, and wash until neutral , dried at 120°C for 10h, and calcined at 700°C for 2h to obtain the product W1.
Embodiment 2
[0039] 22.8gMgSO 4 , 11.4gMg(NO 3 ) 2 ·6H 2 O, 9.58gMgCO 3 , 17.47gAl 2 (SO 4 ) 3 , 40.24gTi(SO 4 ) 2 9H 2 O, 15.15gFe(NO 3 ) 3 9H 2 O, 6.78gCe(NO 3 ) 3 ·6H 2 O dissolved in 200gH 2 O, prepared as solution A, and stirred at room temperature for 4h; 30gNaOH, 10gKOH were dissolved in 200gH 2 In O, configure solution B; add solution A dropwise to solution B heated in a vigorously stirred 60°C water bath, continue stirring for 4 hours after the addition is complete, to obtain slurry C, and keep the pH value of the slurry ≥ 10 during the entire mixing process , Put the slurry C into a reaction kettle lined with polytetrafluoroethylene, crystallize at 90°C for 20h, then cool, filter with suction, wash until neutral, dry at 110°C for 10h, and roast at 700°C for 2h to obtain product W2.
Embodiment 3
[0041] 30.13gMgCl 2 ·6H 2 O, 38.54gC 4 h 6 o 4 Mg·6H 2 O, 16.8g AlCl 3 , 47.12gC 16 h 36 o 4 Ti, 6.77gSr(NO 3 ) 2 , 3.67gZn (NO 3 ) 2 ·6H 2 O was dissolved in 200g of absolute ethanol, prepared as solution A, and stirred at room temperature for 4h; 25gNaOH, 15gKOH, 8gNaCO 3 Soluble in 200gH 2 In O, configure solution B; add solution A dropwise to solution B heated in a vigorously stirred 60°C water bath, continue stirring for 4 hours after the addition is complete, to obtain slurry C, and keep the pH value of the slurry ≥ 10 during the entire mixing process , put the slurry C into a reaction kettle lined with polytetrafluoroethylene, crystallize at 70°C for 17h, then cool, filter with suction, wash until neutral, dry at 110°C for 10h, and roast at 800°C for 2h to obtain product W3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com