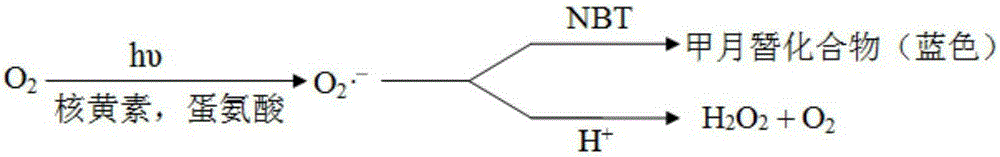
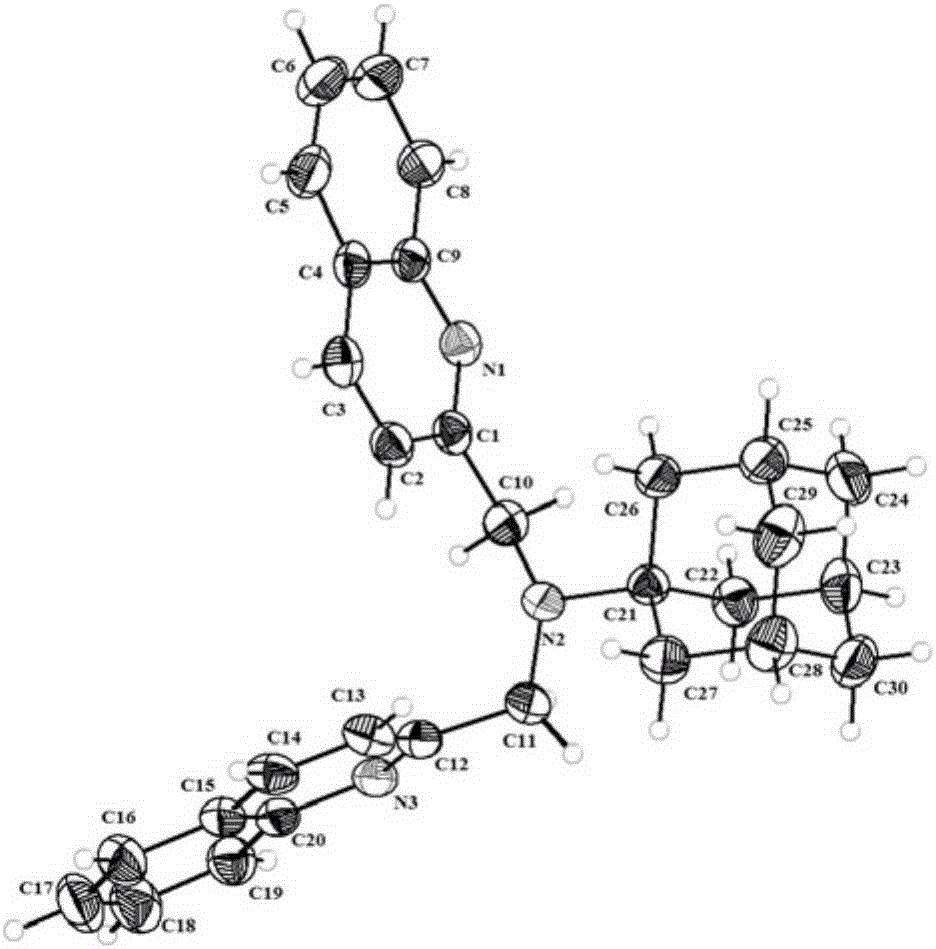
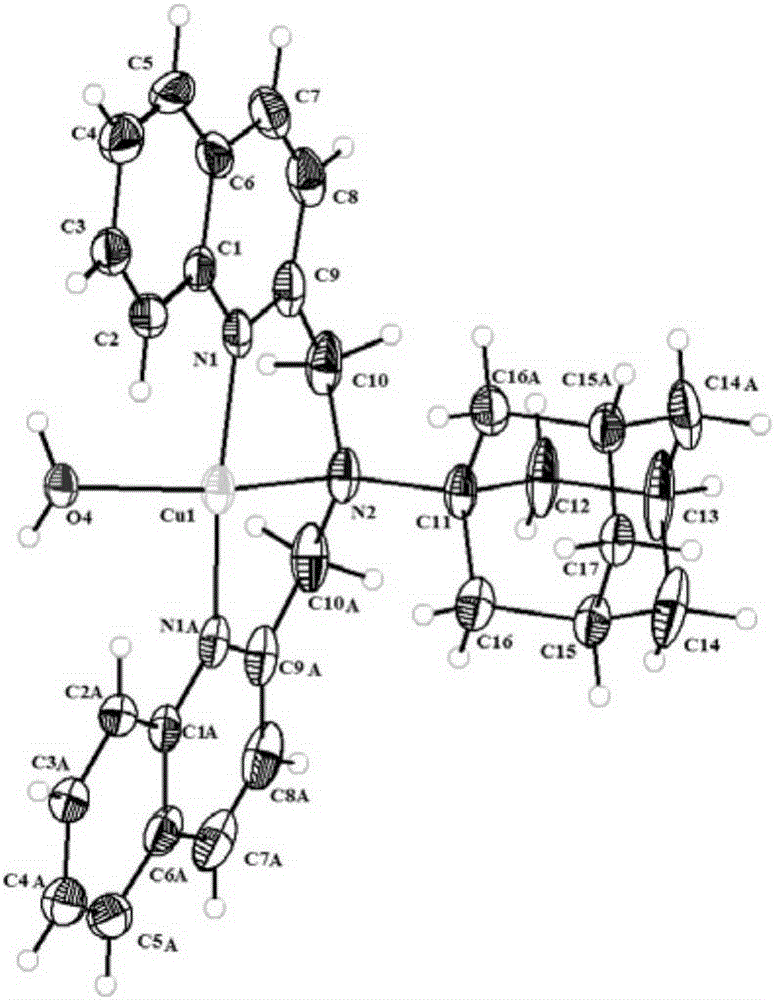
Adamantyl quinoline complexes, intermediates, preparation methods and applications thereof
A technology of adamantyl quinoline and complexes, which is applied in the direction of zinc organic compounds, copper organic compounds, drug combinations, etc., can solve the problems of difficult cell membrane penetration, alloantigenicity, easy hydrolysis by protease, and difficulty in SOD extraction. Achieve strong biocompatibility, strong lipophilicity and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In the preparation method of the above intermediate, in order to increase the reaction speed of the contact reaction, the contact reaction is preferably carried out under heating, and the reaction temperature may be 81-100°C; preferably 85-90°C.
[0042] According to the present invention, in order to make the contact reaction fully proceed, preferably, the reaction time of the contact reaction may be 1-15 h, preferably 2-8 h.
[0043] The method for purifying the crude product of the intermediate obtained through the contact reaction is not particularly limited, and a relatively simple method is to perform pure crystallization of the crude product of the intermediate in absolute ethanol to obtain a pure intermediate.
[0044] The present invention also provides a preparation method for the adamantyl quinoline complex of the structure shown in formula (I) or formula (II), wherein, in the presence of an organic solvent, the Intermediates and [M(N3) 2 ]·6H 2 O, [M(N1 N2) ...
Embodiment 1-1
[0058] The preparation of the intermediate of structure shown in formula (L):
[0059]
[0060] In a 250mL round bottom flask, add 1-adamantanamine hydrochloride solid 5.0mmol, 2-chloromethyl-quinoline hydrochloride 10.05mmol and 60mL anhydrous acetonitrile solvent, and the mixture is magnetically stirred at 20°C, After dissolving, add dry 15.0mmol potassium carbonate solid, reflux at 85°C for 6 hours, cool to 20°C, remove insoluble matter by filtration, rotate the filtrate, and dissolve the obtained crude product in absolute ethanol. Color cubic crystal A1, the yield is 68%.
[0061] The above-mentioned A1 is detected, and the result of elemental analysis is C 30 h 31 N 3 : C, 83.17%; N, 9.56%; H, 7.32%; theoretically calculated values: C, 83.10%; N, 9.69%; H, 7.21%, indicating that the results of elemental analysis are consistent with the results of theoretical calculations. 1 HNMR (CDCl 3 ,300MHz): δ(ppm):7.92-7.99(m,4H,Ar-H-2,3),7.67-7.72(m,4H,Ar-H-5,6),7.60(s,2H,A...
Embodiment 1-2
[0063] The preparation of the intermediate of structure shown in formula (L):
[0064]
[0065] According to the method of Example 1-1, the difference is that the potassium carbonate is 25 mmol, and colorless cubic crystal A2 is obtained with a yield of 69%.
[0066] The above-mentioned A2 is detected, and the result of the elemental analysis is C 30 h 31 N 3 : C, 83.17%; N, 9.56%; H, 7.32%; theoretically calculated values: C, 83.10%; N, 9.69%; H, 7.21%, indicating that the results of elemental analysis are consistent with the results of theoretical calculations. 1 HNMR (CDCl 3 ,300MHz): δ(ppm):7.92-7.99(m,4H,Ar-H-2,3),7.67-7.72(m,4H,Ar-H-5,6),7.60(s,2H,Ar -H-4), 7.42-7.44 (s, 2H, Ar-H-7), 4.21 (s, 4H, CH 2 ), 2.06 (m, 3H, cycl-CH), 1.85 (m, 6H, cycl-CH 2 ), 1.62 (m,6H,cycl-CH 2 ). IR (KBrdisc, cm -1 ): 3059, 2093, 2847, 1605, 1556, 1502, 1456, 1304, 1126, 830, 740, 624.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com