Genetically engineered bacterium for producing alpha-ketobutyric acid and application of genetically engineered bacterium
A technology of genetically engineered bacteria and ketobutyric acid, applied in the biological field, can solve the problems of complex reaction conditions, high production cost, high energy consumption, etc., and achieve the effects of simple fermentation process, low production cost, and high acid production rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
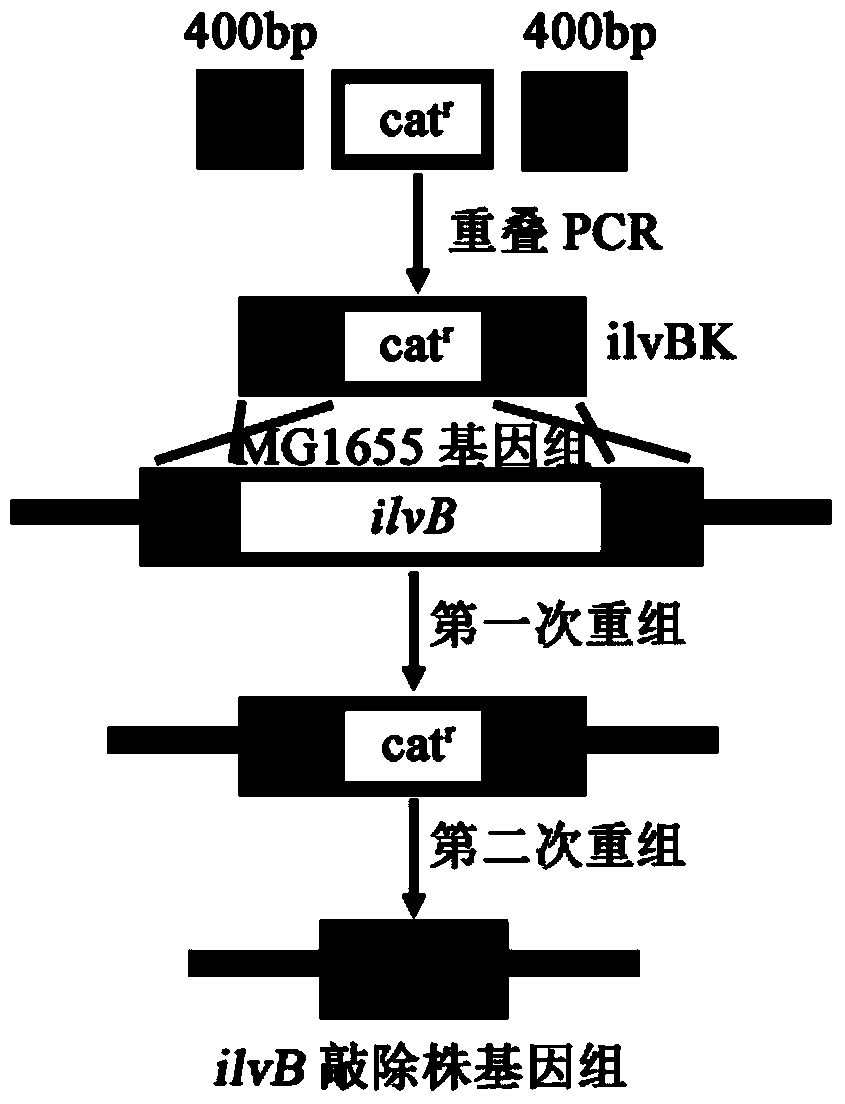
[0063] Example 1: Knockout of the gene ilvB encoding the large subunit of E.coli MG1655 acylhydroxyacid synthase I
[0064] Design primers ilvB1-F, ilvB1-R, ilvB3-F and ilvB3 for the amplification of the upper and lower homology arms according to the 400 bp sequence of the 5' and 3' ends of the ilvB (GeneID: 948181) gene in Escherichia coli MG1655 in the NCBI database -R, and use the bacterial genomic DNA as a template to amplify the homology arm fragment.
[0065] The PCR conditions were 94°C for 5min for one cycle, 94°C for 30s, 55°C for 30s, 72°C for 40s for 30 cycles, and 72°C for 10min as one cycle, and the reaction system was 100 μL.
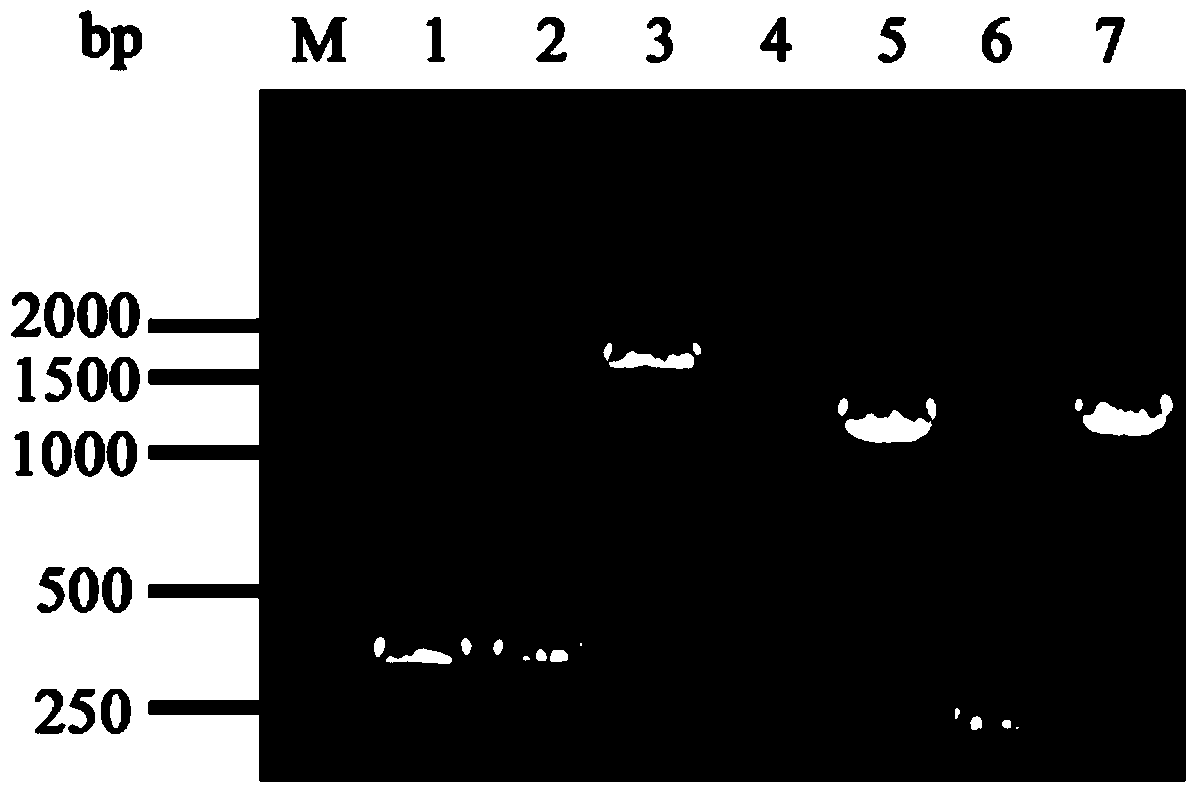
[0066] The PCR products were subjected to 1.5% agarose gel electrophoresis and then gel-cut to recover, and the obtained fragments were named ilvB1 and ilvB3, respectively.
[0067] Amplification primers ilvB2-F and ilvB2-R were designed according to the sequence of the chloramphenicol resistance gene cassette in the plasmid pKD3, and the...
Embodiment 2
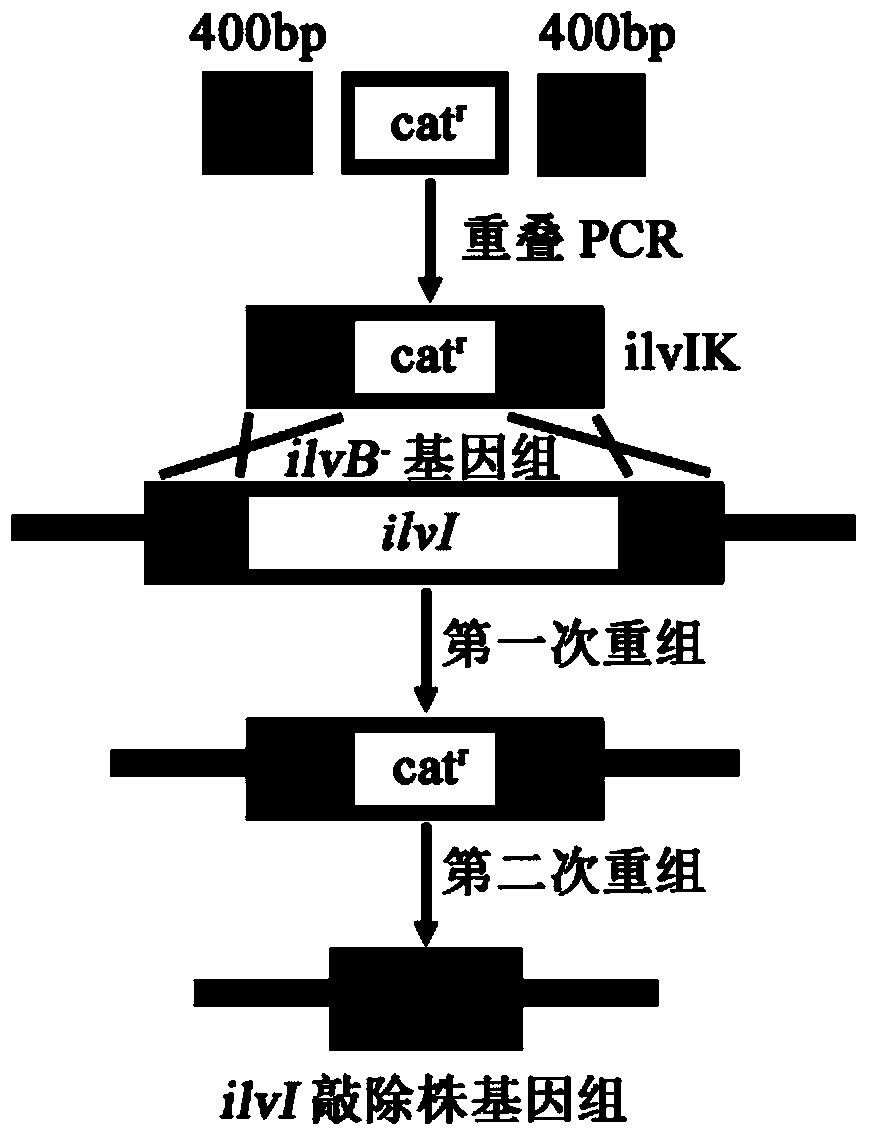
[0077] Example 2: Knockout of the gene ilvI encoding the large subunit of acetohydroxyacid synthase III in E.coli MG1655 ilvB knockout strain
[0078] Homology arm amplification primers ilvI1-F, ilvI1-R, ilvI3-F and ilvI3-R were designed according to the 5' and 3' end 400bp sequences of the Escherichia coli (Escherichia coli) MG1655ilvI (GeneID: 947267) gene in the NCBI database. The genome DNA of the bacterium was used as a template to amplify the homology arm fragment.
[0079] The PCR conditions were 94°C for 5min for one cycle, 94°C for 30s, 55°C for 30s, 72°C for 40s for 30 cycles, and 72°C for 10min as one cycle, and the reaction system was 100 μL.
[0080] Amplification primers ilvI2-F and ilvI2-R were designed according to the sequence of the chloramphenicol resistance gene cassette in the plasmid pKD3, and the fragment of the chloramphenicol resistance gene cassette was amplified using the plasmid as a template. The PCR conditions were 94 ° C for 5 min for 1 cycle, 9...
Embodiment 3
[0086] Construction of embodiment 3THRZ bacterial strain
[0087] According to the 5' and 3' end 400bp sequences of thrL (GeneID: 948283) gene in E. coli MG1655 in the NCBI database, the homology arm amplification primers thrL1-F, thrL1-R, thrL3-F and thrL3-R were designed, and The homology arm fragment was amplified using the bacterial genome DNA as a template.
[0088] The PCR conditions were 94°C for 5min for one cycle, 94°C for 30s, 55°C for 30s, 72°C for 40s for 30 cycles, and 72°C for 10min as one cycle, and the reaction system was 100 μL.
[0089] The PCR products were electrophoresed on a 1.5% agarose gel and recovered by cutting the gel, and the obtained fragments were named thrL1 and thrL3, respectively.
[0090] Amplification primers thrL2-F and thrL2-R were designed according to the sequence of the chloramphenicol resistance gene cassette in the plasmid pKD3, and the fragment of the chloramphenicol resistance gene cassette was amplified using the plasmid as a temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com