Method for treating chromium-containing alkali solution
An alkaline aqueous solution and solution technology, applied in chemical instruments and methods, water/sewage multi-stage treatment, water/sludge/sewage treatment, etc., can solve the problems of unrecoverable alkali and high acid consumption, and achieve low production cost, Good economic benefit and avoid secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
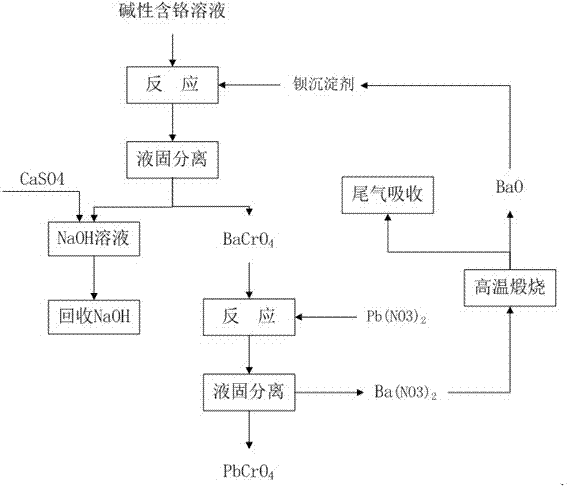
[0020] Example 1: figure 1 Shown, the method for this processing chromium-containing alkaline aqueous solution adopts following processing steps.
[0021] (1) Weigh the solid Ba(OH) according to the molar ratio Cr / Ba=0.8 2 Add 0.4g to 100mL of 1# chromium-containing alkaline aqueous solution (basic chromium-containing solution), which contains chromium 6 + =1g / L, NaOH=100g / L, turn on the stirring, the reaction temperature is 20°C, and the reaction time is 30min.
[0022] (2) At the end of the reaction, suction filtration is performed to obtain barium chromate precipitate and a solution containing sodium hydroxide; the content of liquid phase chromium analyzed by chemistry and instruments is 8 mg / L.
[0023] (3) Barium chromate precipitation Add Pb (NO 3 ) 2 The solution is reacted, and the lead chromate precipitate and the solution containing barium nitrate are obtained through liquid-solid separation;
[0024] (4) Evaporate the solution containing barium nitrate and roa...
Embodiment 2
[0025] Embodiment 2: The method for processing the chromium-containing alkaline aqueous solution adopts the following processing steps.
[0026] (1) Weigh the solid Ba(OH) according to the molar ratio Cr / Ba=1 2 Add 0.6g into 100mL1# chromium-containing alkaline aqueous solution, which contains chromium 6 + =1g / L, NaOH=100g / L, turn on the stirring, the reaction temperature is 50°C, and the reaction time is 30min.
[0027] (2) At the end of the reaction, suction filtration is performed to obtain a barium chromate precipitate and a solution containing sodium hydroxide; the content of liquid phase chromium is 5 mg / L by chemical and instrumental analysis.
[0028] (3) Barium chromate precipitation Add Pb (NO 3 ) 2 The solution is reacted, and the lead chromate precipitate and the solution containing barium nitrate are obtained through liquid-solid separation;
[0029] (4) Barium oxide is obtained by evaporating the solution containing barium nitrate and roasting at 650°C.
Embodiment 3
[0030] Embodiment 3: The method for processing the chromium-containing alkaline aqueous solution adopts the following processing steps.
[0031] (1) Weigh the solid BaCl according to the molar ratio Cr / Ba=1.5 2 Add to 100mL 2# chromium-containing alkaline aqueous solution, which contains chromium 6 + =2g / L, NaOH=200g / L, turn on the stirring, the reaction temperature is 100°C, and the reaction time is 30min.
[0032] (2) reaction end point, suction filtration, obtain barium chromate precipitation and containing sodium hydroxide solution; Adopt the content of chemical and instrument analysis liquid phase chromium to be 8mg / L; Contain sodium hydroxide solution can directly reclaim, directly use in Hydrothermal alkaline hydrometallurgical process or directly used for flue gas desulfurization ingredients.
[0033] (3) Barium chromate precipitation Add Pb (NO 3 ) 2 The solution is reacted, and the lead chromate precipitate and the solution containing barium nitrate are obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com