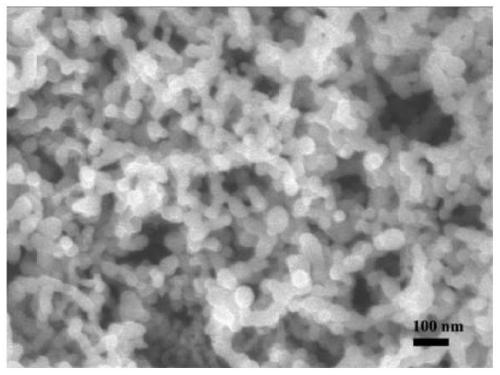
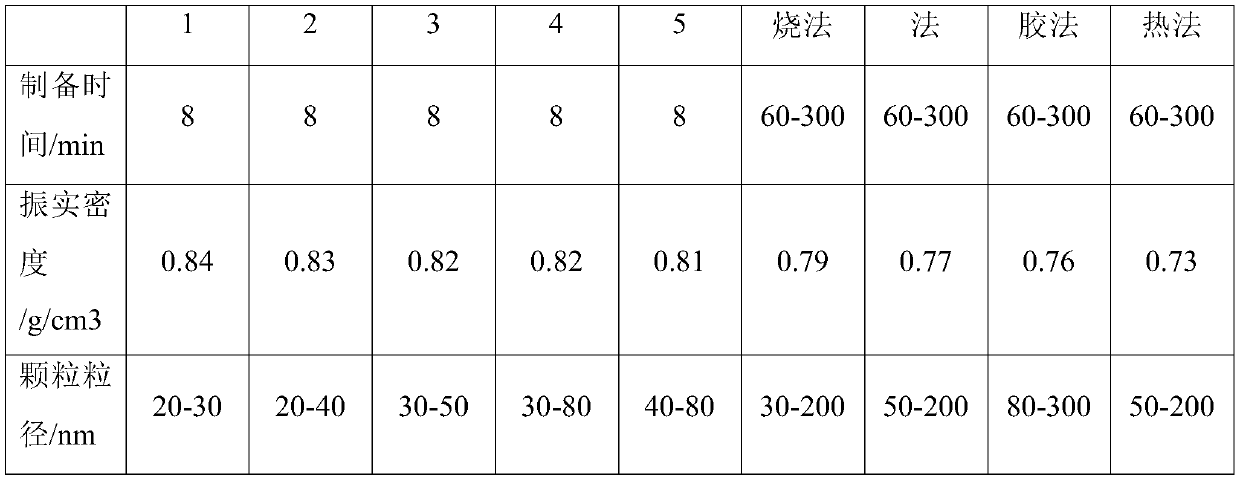
Nano lithium iron phosphate and preparation method thereof
A nano-iron phosphate, ferric salt technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of irregular iron phosphate particles, poor performance of lithium iron phosphate, low tap density, etc. Achieve the effect of short hydrothermal time, shortened sample preparation time and high tap density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of nano iron phosphate, comprising the following steps:
[0039] (1) Weigh ferric chloride and ammonium phosphate in equimolar amounts, place them in a 50mL microwave digestion tank, stir for 10min and then ultrasonicate for 10min to obtain solution A;
[0040] (2) adding sodium dodecylbenzenesulfonate of ferric chloride mass 1% to solution A to obtain solution B;
[0041] (3) Stir the solution B for 30 minutes, then sonicate it for 30 minutes, place it in a microwave digestion apparatus, set the temperature at 110°C, and take it out for 8 minutes, and take it out after cooling to room temperature to obtain solid A;
[0042] (4) After several times of suction filtration with deionized water, put it in an oven and dry it at 60°C for 2 hours. After drying, place it in a tube furnace for calcination to improve the crystallinity of the material. After 1 hour, nitrogen gas was introduced as a protective gas during the calcination process, and then nanom...
Embodiment 2
[0045] A preparation method of nano iron phosphate, comprising the following steps:
[0046] (1) Weigh ferric chloride and ammonium phosphate in equimolar amounts, place them in a 50mL microwave digestion tank, stir for 10min and then ultrasonicate for 10min to obtain solution A;
[0047] (2) adding sodium dodecylbenzenesulfonate of ferric chloride mass 1% to solution A to obtain solution B;
[0048] (3) Stir the solution B for 30 minutes, then sonicate it for 30 minutes, place it in a microwave digestion apparatus, set the temperature at 90°C for 8 minutes, take it out after cooling to room temperature, and obtain solid A;
[0049] (4) Suction filter solid A several times with deionized water and dry it in an oven at 60°C for 2 hours. After drying, place it in a tube furnace for calcination to improve the crystallinity of the material. °C, time 1h, nitrogen gas is passed as a protective gas during the calcination process, and then nanometer iron phosphate is obtained.
[00...
Embodiment 3
[0052] A preparation method of nano iron phosphate, comprising the following steps:
[0053] (1) Weigh ferric chloride and ammonium phosphate in equimolar amounts, place them in a 50mL microwave digestion tank, stir for 10min and then ultrasonicate for 10min to obtain solution A;
[0054] (2) adding sodium dodecylbenzenesulfonate of ferric chloride mass 1% to solution A to obtain solution B;
[0055] (3) Stir the solution B for 30 minutes, then sonicate it for 30 minutes, place it in a microwave digestion apparatus, set the temperature at 130°C, and take it out for 8 minutes, and take it out after cooling to room temperature to obtain solid A;
[0056] (4) Suction filter solid A several times with deionized water and dry it in an oven at 60°C for 2 hours. After drying, place it in a tube furnace for calcination to improve the crystallinity of the material. °C, time 1h, nitrogen gas is passed as a protective gas during the calcination process, and then nanometer iron phosphate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com