Rabies virus ERA attenuated vaccine mutant strain as well as preparation method and live rabies vaccine
A technology of rabies virus and attenuated vaccine, which is applied in the biological field and can solve problems such as animal diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
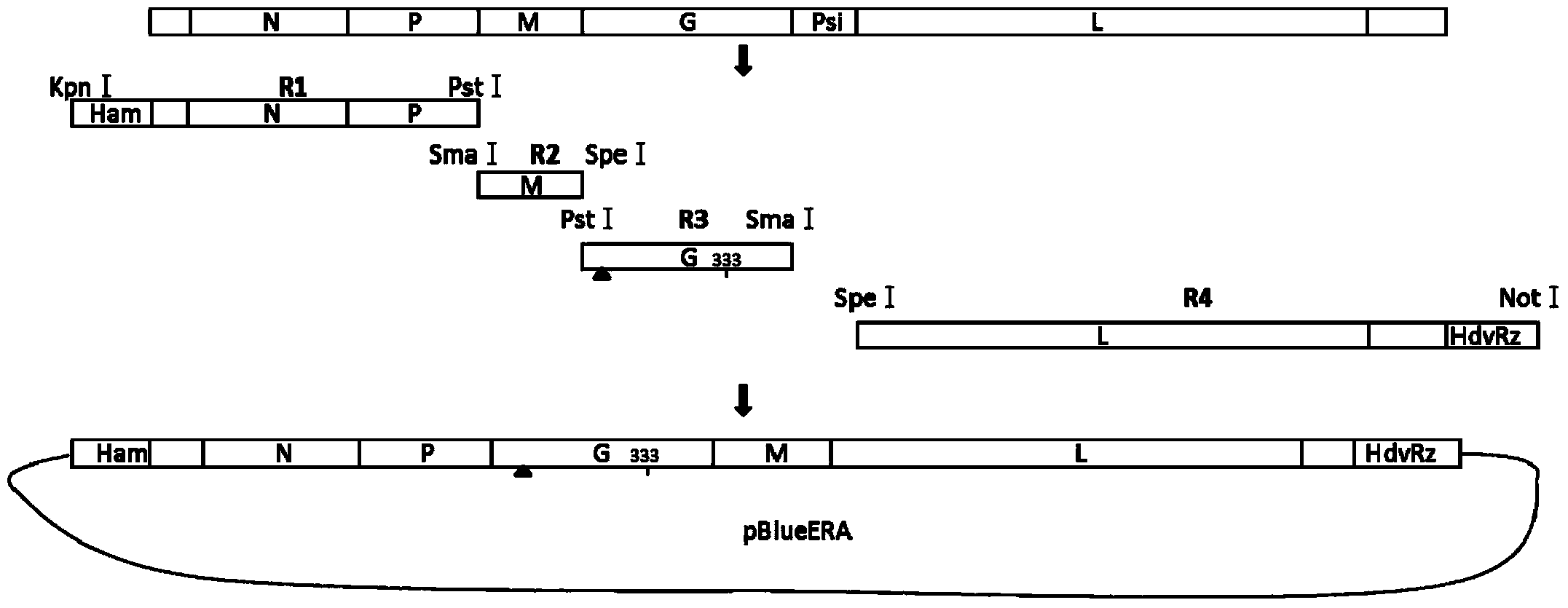
[0046] Reverse genetics plasmid construction
[0047] According to the ERA sequence in Genebank, primers were designed, and the full-length sequence was synthesized in four segments (R1-R4). Hammerhead ribozyme (Ham) was introduced into R1, G protein signal peptide partial deletion and 333 amino acid mutation were introduced into R3, and hepatitis D ribozyme was introduced into R4.
[0048] 1F: GTCACT GGTAAC TGG TGTTAAGCGTCTGATGAGTCCGTGAGGACG
[0049] AAACTATAGGAAAGGAATTCCTATAGTC ACGCTTAACAACCAGATCAAAG
[0050] (KpnI / Ham) (SEQ ID NO: 3)
[0051] 1R: CTGCAG GTTTTTTTCATG (PstI) (SEQ ID NO: 4)
[0052] 2F: CCCGGG AGGCAACACC (Sam I) (SEQ ID NO: 5)
[0053] 2R: ACTAGT TAATAGTTTTTTTCAC (SpeI) (SEQ ID NO: 6)
[0054] 3F: CTGCAG AACATCCTCAAAAGACTCAAGGAAAGATGCAGGCTCTC (PstI) (SEQ ID NO: 7)
[0055] 3R: CCCGGG GTTTTTTTTCAAAAAGAACCCCCC (Sam I) (SEQ ID NO: 8)
[0056] 4F: ACTAGT CATTAGATCAGAAG (SpeI) (SEQ ID NO: 9)
[0057] 4R: GCGGCCGC GCCCTCCCTTAGCCATCCGAGTGGG...
Embodiment 2
[0066] virus recovery
[0067] When BHK-21 cells were cultured in MEM complete medium with 10% newborn bovine serum to 90% confluence in a 6-well plate, the liposome 2000 method was used to co-transfect the following plasmids: 4ug / well of pBlueERA and 4 kinds of helper plasmids pBlueN( 2ug / well), pBlueP (lug / well), pBlueL (lug / well), pT7 (4ug / well). After transfection, the cells were placed in 5% CO 2 Discard the supernatant after incubating at 37°C for 4-6 hours in an incubator, supplement MEM complete medium with 5% newborn bovine serum, and incubate at 37°C, 5% CO 2 After culturing in the incubator for 72 hours, the cells were frozen and thawed three times, the supernatant was collected by centrifugation, and the recovered virus was obtained by filtering with a filter membrane with a particle size of 0.22 μm.
Embodiment 3
[0069] Titration of virus after recovery
[0070] BHK-21 cells were cultured in a 24-well plate to a single layer, and the recovered virus was diluted 10 times to infect the BHK-21 cells. 37℃5%CO 2 After culturing in an incubator for 72 hours, fix in 80% acetone at -20°C for half an hour, wash with PBS and stain with FITC-labeled rabies virus N protein antibody at 37°C for 60 minutes. Fluorescent wells were counted with a fluorescence microscope after washing with PBS. The results showed that the virus was amplified in BHK-21 cells after recovery, and the virus titer reached 6×10 7 TCID 50 / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com