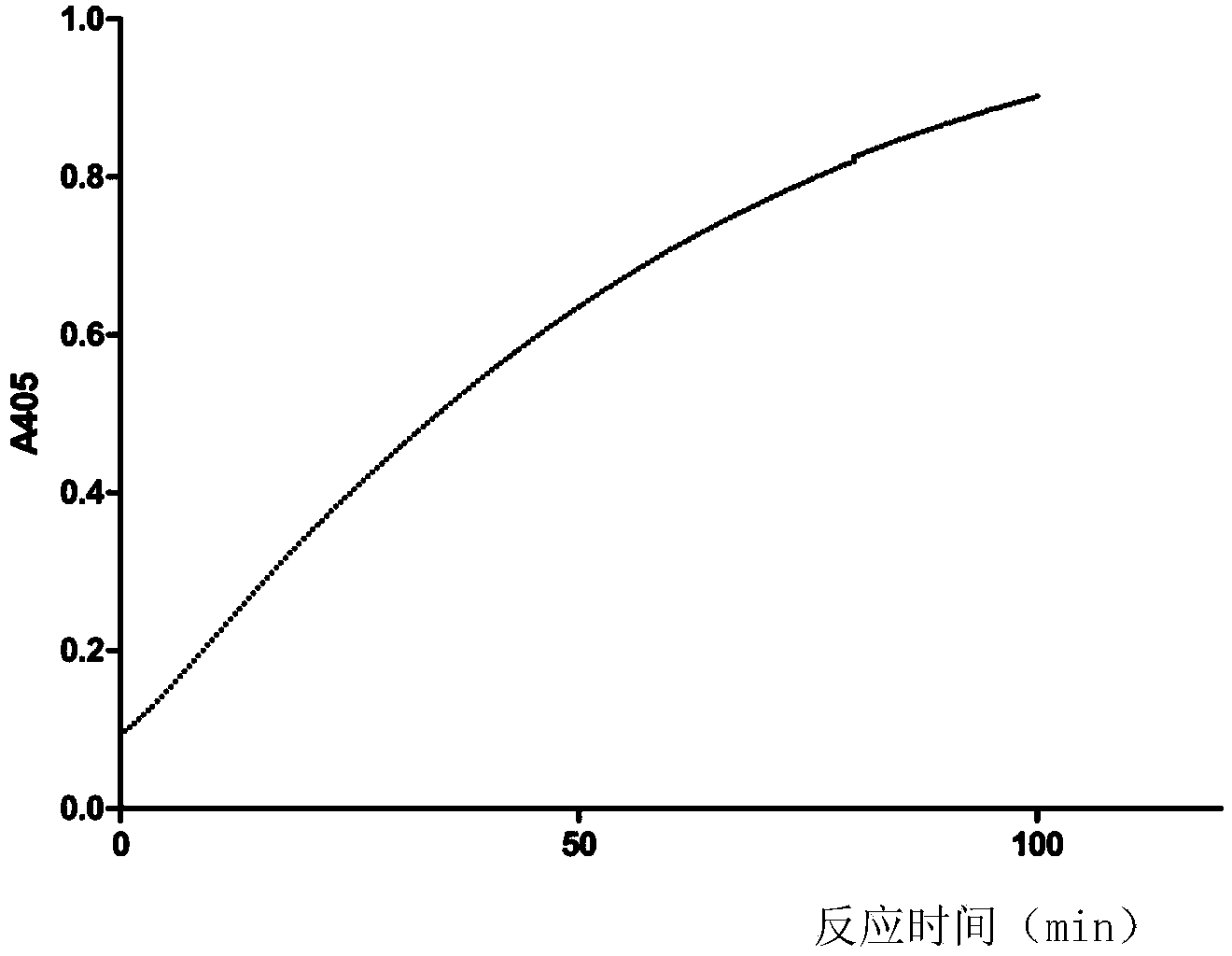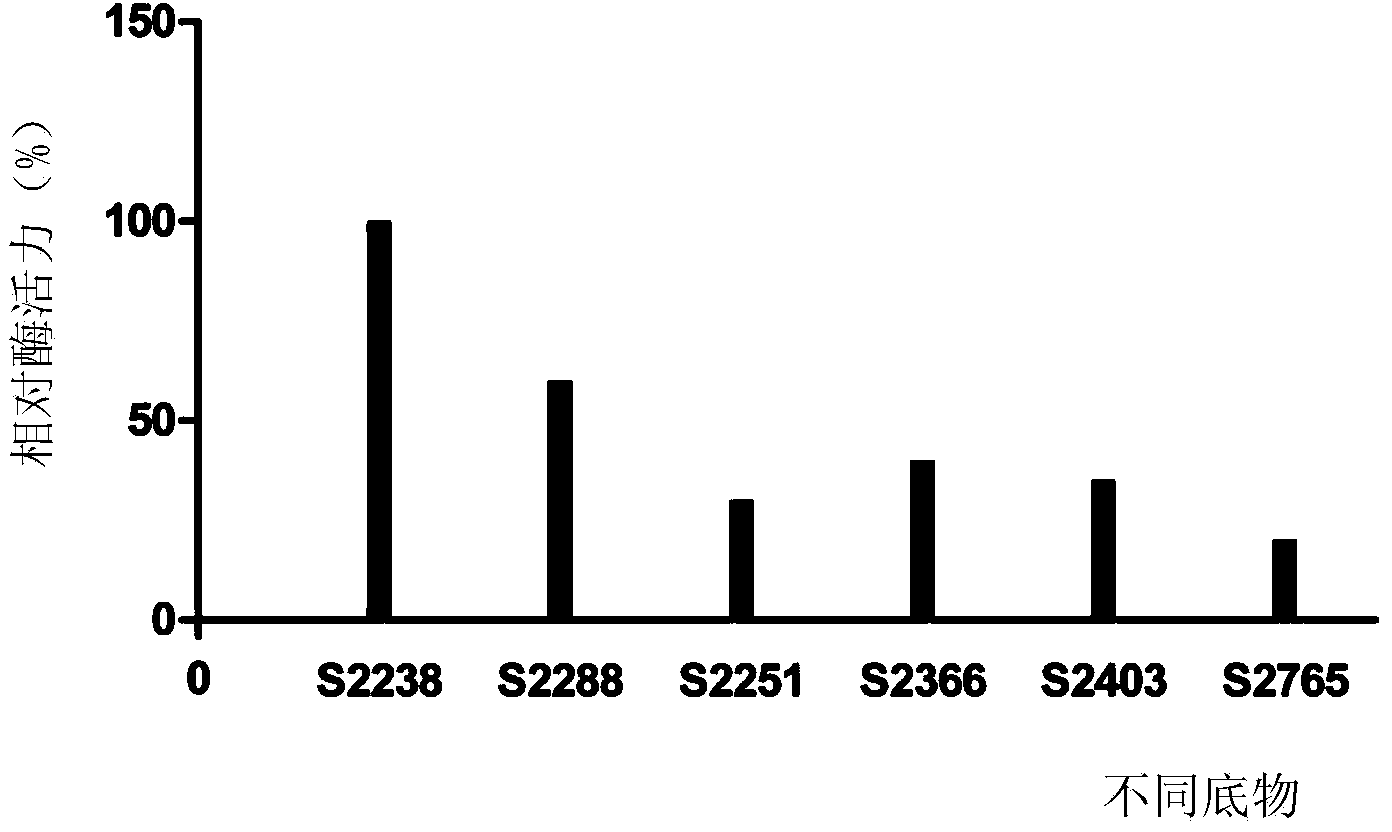Method for determining thrombin-like enzyme activity
A technology similar to thrombin and activity, applied in the field of medical testing, can solve the problems of subjective interference of the tester, long time consumption, linearity, sensitivity, poor accuracy, etc., achieve the effect of simple and unified reagent source, simple operation process, and avoid reagent manufacturers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 The selection of standing time and reaction time in assay method of the present invention
[0039] In this example, the recombinant site-directed mutant batroxobin (ZL200710011566.4) was used as the thrombin-like sample to be tested, and the resting time and reaction time in the activity determination method were screened.
[0040] Prepare 2mmol / L S-2238 (chromogenix, Italy) substrate aqueous solution with deionized water. The recombinant site-directed mutation batroxobin with batch number 20100101 produced by Nuokang Biotechnology Co., Ltd. was randomly selected as the test sample, and the sample was diluted to 100 nmol / L with 50 mmol / L Tris-HCl pH7.4.
[0041] Add 60 μL recombinant batroxobin dilution solution for site-directed mutagenesis, 890 μL 50 mmol / L Tris-HCl, and 50 μL 2 mmol / L S2238 aqueous solution into a 1 mL cuvette, and mix well. Put the cuvette into a 37°C UV spectrophotometer (thermo, evolution220, USA), and immediately measure the absorban...
Embodiment 2
[0046] Example 2 The selection of substrate in assay method of the present invention
[0047] In this example, the recombinant site-directed mutant batroxobin (ZL200710011566.4) was used as the thrombin-like sample to be tested, and the substrates in the activity assay method were screened.
[0048] Prepare 2 mmol / L aqueous solutions of S2238, S2288, S2251, S2366, S2403, and S2765 substrates with deionized water. The recombinant site-directed mutation batroxobin with batch number 20100101 produced by Nuokang Biotechnology Co., Ltd. was randomly selected as the test sample, and the sample was diluted to 100 nmol / L with 50 mmol / L Tris-HCl pH7.4.
[0049] Add recombinant site-directed mutagenesis batroxobin dilution solution to 1mL cuvette, add 50 μL of the above-mentioned 2mmol / L S2238, S2288, S2251, S2366, S2403, S2765 substrate aqueous solution, then supplement the Tris-HCl to 1mL, mix well, The batroxobin had a final concentration of 1-17 nmol / L, stood at 37° C. for 5 minu...
Embodiment 3
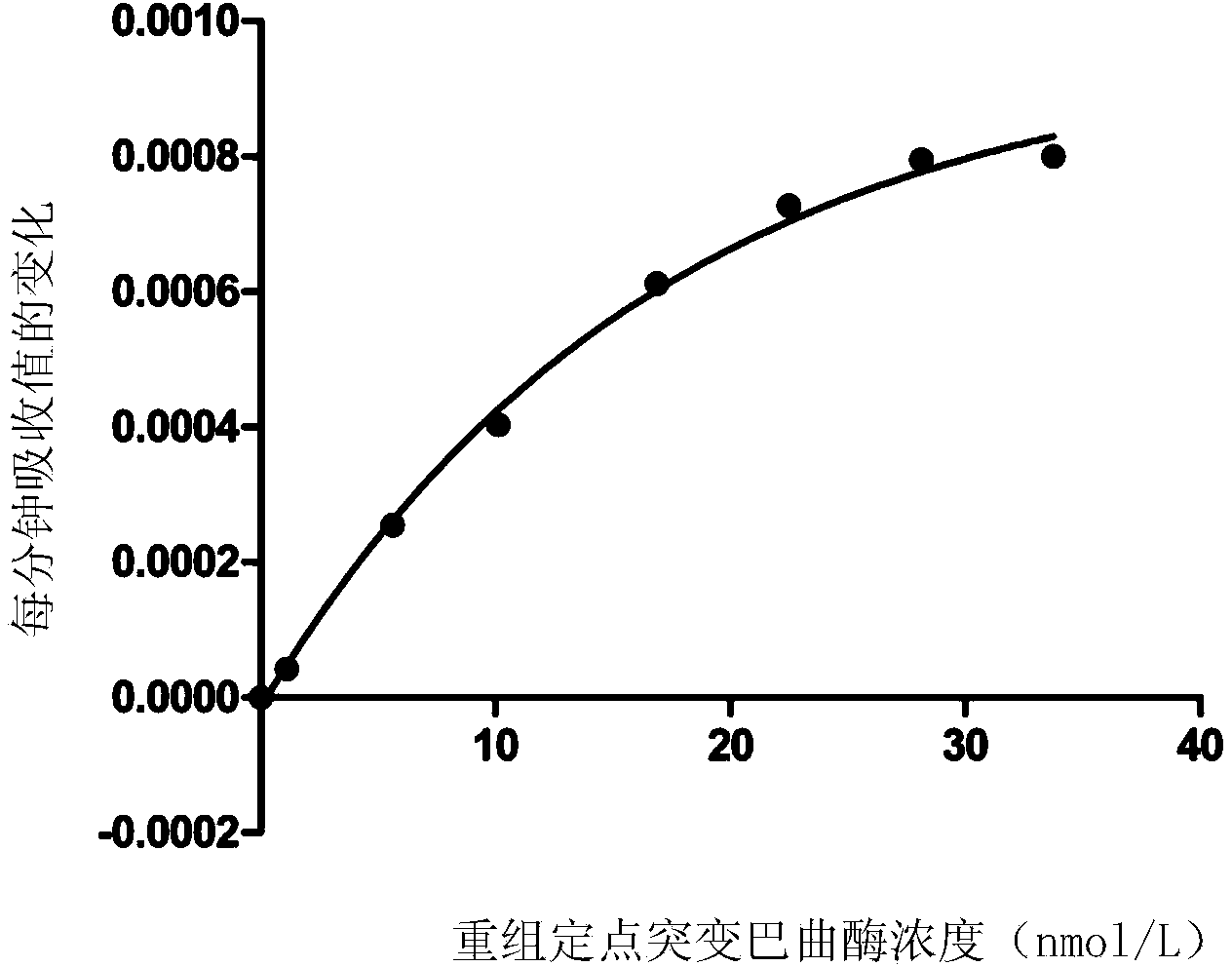
[0051] Example 3 Selection of the linear range of the final concentration of the enzyme sample to be tested in the assay method of the present invention
[0052] In this example, the recombinant site-directed mutant batroxobin (ZL200710011566.4) was used as the thrombin-like sample to be tested, and the linear range of the final concentration in the activity determination method was screened.
[0053] The recombinant site-directed mutation batroxobin enzyme with batch number 20100101 produced by Nuokang Biotechnology Co., Ltd. was randomly selected as a test sample, and diluted to 100 nmol / L with 50 mmol / L Tris-HCl pH7.4. Add different volumes of the enzyme dilution solution to be tested, Tris-HCl buffer and chromogenic substrate to obtain a 1 mL reaction system, as shown in Table 1.
[0054] Table 1 1mL reaction system with different compositions
[0055]
[0056]
[0057] Put the above different reaction systems into a UV spectrophotometer at 37°C and let it stand f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com