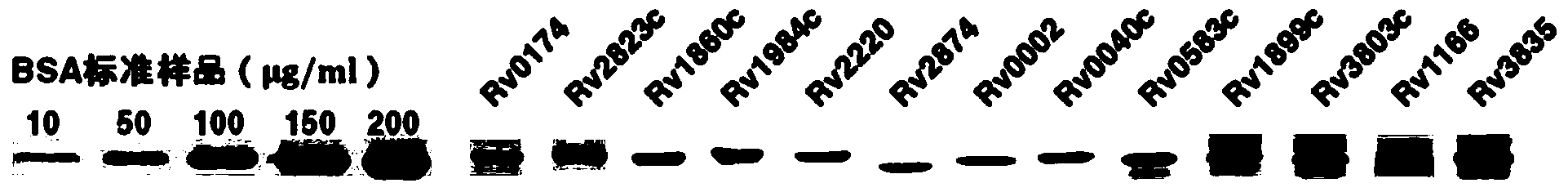Mycobacterium M. tuberculosis holoprotein chip and application thereof
A technology of mycobacterium tuberculosis and combined mycobacteria, applied in the biological field, can solve the problems of high GC content in the genome, difficult clinical prevention, slow growth, etc., and achieve the effect of improving research efficiency, simplifying research design and operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
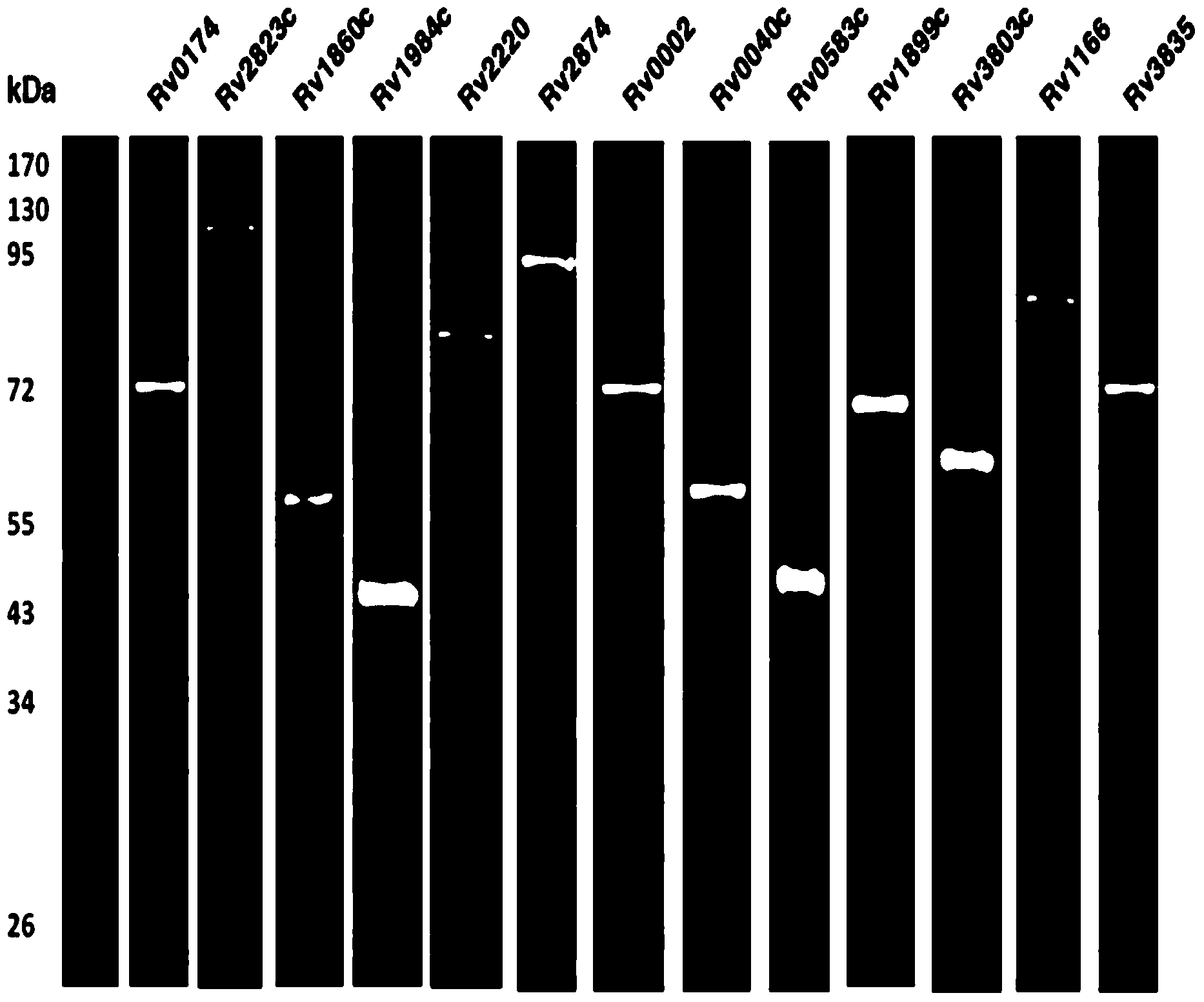
[0035] Embodiment 1, the preparation of whole protein chip
[0036] (1) High-throughput preparation of Mycobacterium tuberculosis proteins
[0037] 1. Express
[0038] The genetically engineered Saccharomyces cerevisiae uses galactose to induce overexpression, and the specific process is as follows:
[0039] First from the Mycobacterium M.tuberculosis H37Rv strain (Beijing strain) (Nature1998Nov12;396(6707):190; the public can obtain from Institute of Biophysics, Shanghai Jiao Tong University, Chinese Academy of Sciences Wuhan Virus Research Institute, Guangdong Tibikang Biotechnology Co., Ltd.) and CDC1551 strain (J Bacteriol.2002October;184(19):5479–5490; the public can obtain from Guangdong Tibikang Biotechnology Co., Ltd..), respectively 3749 proteins of H37Rv strain and 419 protein gene coding fragments of CDC1551 strain were obtained by PCR cloning, a total of 4168 proteins, and the gene coding fragments were connected to the pDONR221 vector (purchased from Invitrogen)...
Embodiment 2
[0200] Embodiment 2, application whole protein chip detects serum
[0201] (1) Preparation of serum samples to be tested
[0202] The serum of tuberculosis patients (infected with Mycobacterium tuberculosis) and the serum of healthy people (Guangdong Provincial Tuberculosis Control Center) were left at room temperature for 2 hours or overnight at 4°C and then centrifuged at 1000g for about 20 minutes. The supernatant can be tested immediately; or aliquot , and store the specimens at -20°C or -80°C, but repeated freezing and thawing should be avoided. Samples thawed at 4°C should be centrifuged again before testing.
[0203] (2) Preparation of various buffers and reagents involved in the diagnostic process
[0204] (1) Sample diluent (see Table 9): pH7.4 PBS solution
[0205] Table 9
[0206]
[0207] (2) Washing solution (see Table 10): PBST solution with pH 7.4
[0208] Table 10
[0209]
[0210] (3) Chip blocking solution (see Table 11): pH 7.4 PBS solution conta...
Embodiment 3
[0228] Example 3, the whole protein chip interacts with the small molecule compound c-di-GMP
[0229] c-di-GMP (cyclic diguanylic acid) is a ubiquitous second messenger molecule in bacteria that regulates diverse cellular events such as biofilm formation, virulence, motility and cell differentiation. Recent studies have found that Mycobacterium Tuberculosis and Mycobacterium smegmatis have enzymes that synthesize c-di-GMP and regulate c-di-GMP changes, but how c-di-GMP is regulated in Mycobacterium Tuberculosis has not been clear. Using the TB whole protein chip, c-di-GMP interacting proteins can be found globally, and their functions can be studied, so as to understand the role of c-di-GMP in Mycobacterium Tuberculosis more clearly and accurately.
[0230] 1. Closed chip
[0231] Prepare 20 mL of 3% BSA blocking solution with 1 × TBST, pour it into the sealing box, take two whole protein chips prepared in Example 1 from the -80°C refrigerator, put it in it, and put the seali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com