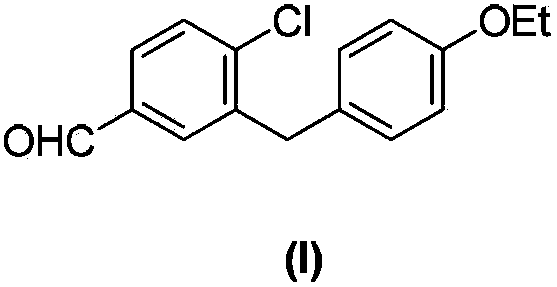
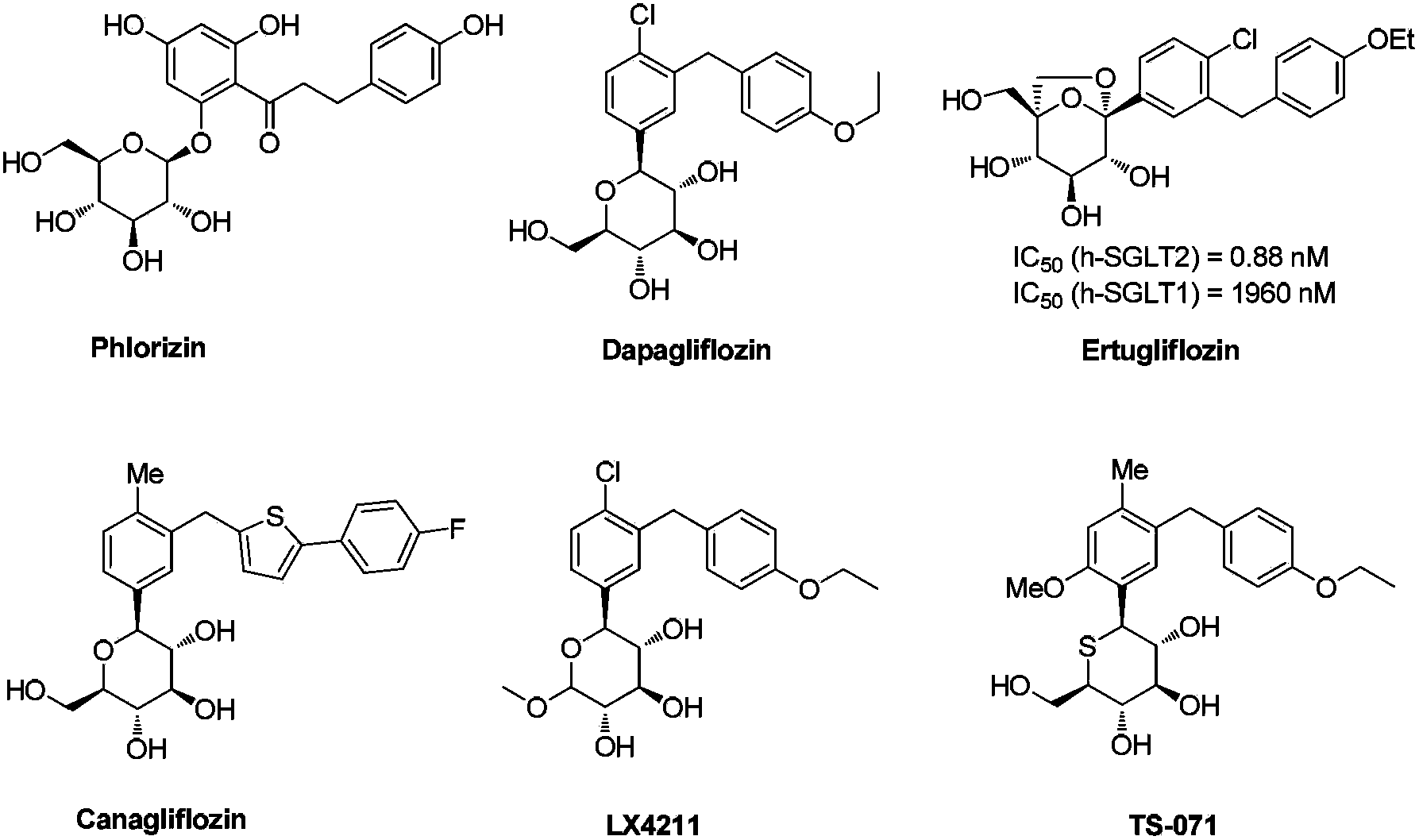
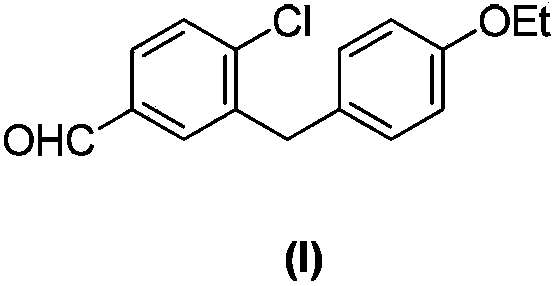
Preparation method of 4-chloro-3-(4-ethoxybenzyl)benzaldehyde
A technology of ethoxybenzyl and benzaldehyde, applied in the field of compound preparation, can solve the problems of difficult separation, expensive starting material of formula 1, etc., and achieve the effects of simple synthesis route, good yield, and cheap and easily available reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Preparation of 5-bromo-2-chlorobenzoyl chloride (compound of formula 7)
[0055]
[0056] Under ice-cooling, add 2-chloro-5-bromobenzoic acid (50g, 0.212mol) (compound of formula 6) into dichloromethane (250ml), add N,N-dimethylformamide (2.5ml), drop Add oxalyl chloride (25ml, 0.255mol), stir at room temperature for 12 hours, TLC (thin layer chromatography) detects that the reaction is complete, spin the reaction solution to obtain 55g of crude product (compound of formula 7).
Embodiment 2
[0057] Embodiment 2: Preparation of 5-bromo-2-chlorobenzoyl chloride (compound of formula 7)
[0058]
[0059] Add 2-chloro-5-bromobenzoic acid (50g, 0.212mol) (compound of formula 6) into thionyl chloride (SOCl 2 ) (500ml), heated to 70°C, reacted for 2 hours, and spin-dried the reaction solution to obtain 55g of crude product.
Embodiment 3
[0060] Embodiment 3: Preparation of (5-bromo-2-chlorophenyl) (4-ethoxyphenyl) ketone (compound of formula 8)
[0061]
[0062] The crude compound of formula 7 (55g) was dissolved in dichloromethane, cooled to -5°C, added phenetole (24ml, 0.19mol), and added aluminum chloride (28.5g, 0.212mol) in batches, keeping the temperature below 0 °C, react for 3h. The reaction solution was poured into ice water, extracted with dichloromethane, washed with 1M (molar concentration) aqueous hydrochloric acid solution, 1M (molar concentration) aqueous sodium hydroxide solution, water, and saturated brine, dried, filtered to remove the desiccant, and spin-dried , recrystallized from ethanol to give a white solid (51 g, 71%).
[0063] Compound of formula 8: 1 H NMR (400MHz, CDCl 3 ): δ1.43-1.46(t,3H),4.08-4.13(m,2H),6.92-6.94(m,2H),7.32-7.33(d,1H),7.47-7.48(d,1H),7.52 -7.54(m,1H),7.75-7.78(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com