Method used for direct production of methyl methoxyacetate via gas-phase carbonylation of methyl aldehyde with methyl alcohol
A technology of methyl methoxyacetate and methanol, which is applied in chemical instruments and methods, carbon monoxide or formate reaction preparation, organic compound preparation, etc. Corrosion reaction equipment and other problems, to achieve easy separation, shorten the process, and avoid the effect of corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
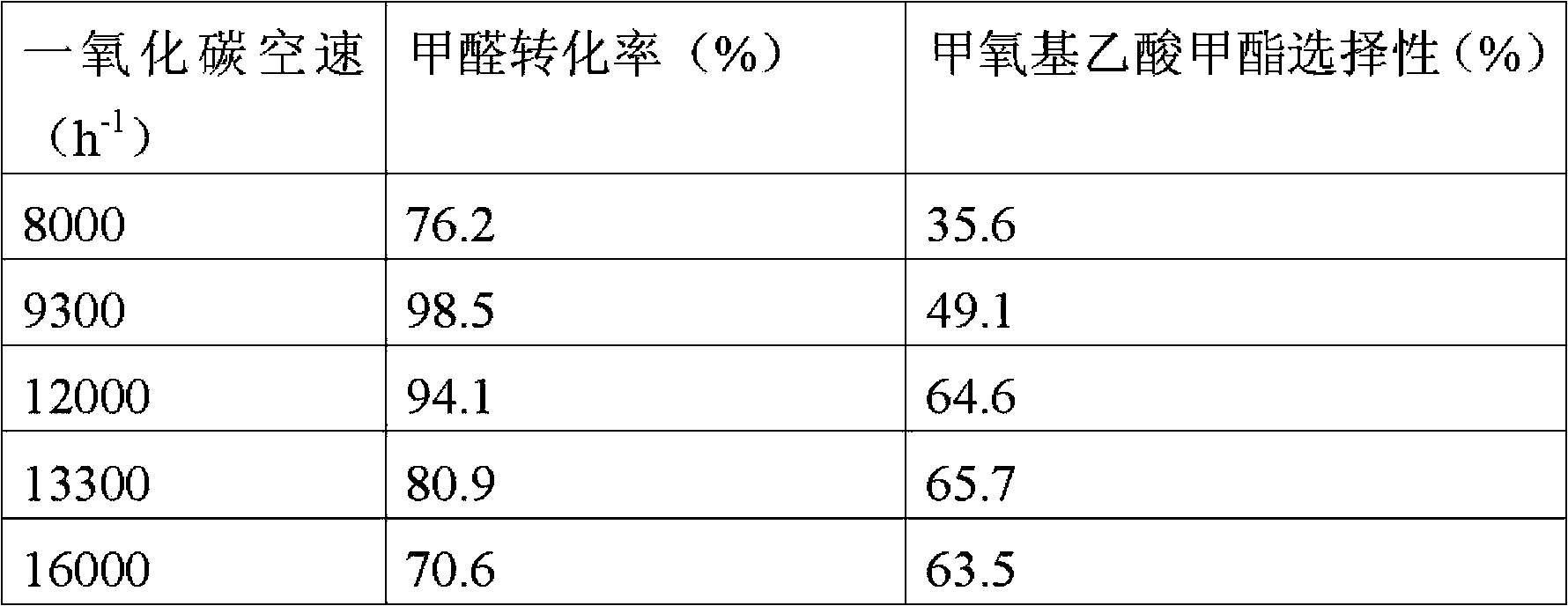
[0023] The 40-60 mesh catalyst obtained by pressing H-ZSM-5 (Si / Al=25) molecular sieve powder into tablets, crushing and sieving. Put 0.6g of catalyst into a reactor with an inner diameter of 8mm, raise the temperature to 500°C in a nitrogen atmosphere, and keep the temperature constant for 1h, then naturally cool down to 140°C. Feed CO into the reaction tube to raise the pressure to 3.0MPa. Methanol and paraformaldehyde (decomposed to formaldehyde under acid catalysis) are carried by CO gas flow, and the saturated vapor pressures of methanol and paraformaldehyde (decomposed to formaldehyde under acid catalysis) are controlled by temperature. The partial pressure ratio of carbon monoxide and methanol is 30, the partial pressure ratio of carbon monoxide and paraformaldehyde is 180, and the total space velocity of carbon monoxide is 10600h -1 . The reactants were analyzed by online chromatography, the conversion rate of formaldehyde was 73.6%, and the selectivity of methyl met...
Embodiment 2
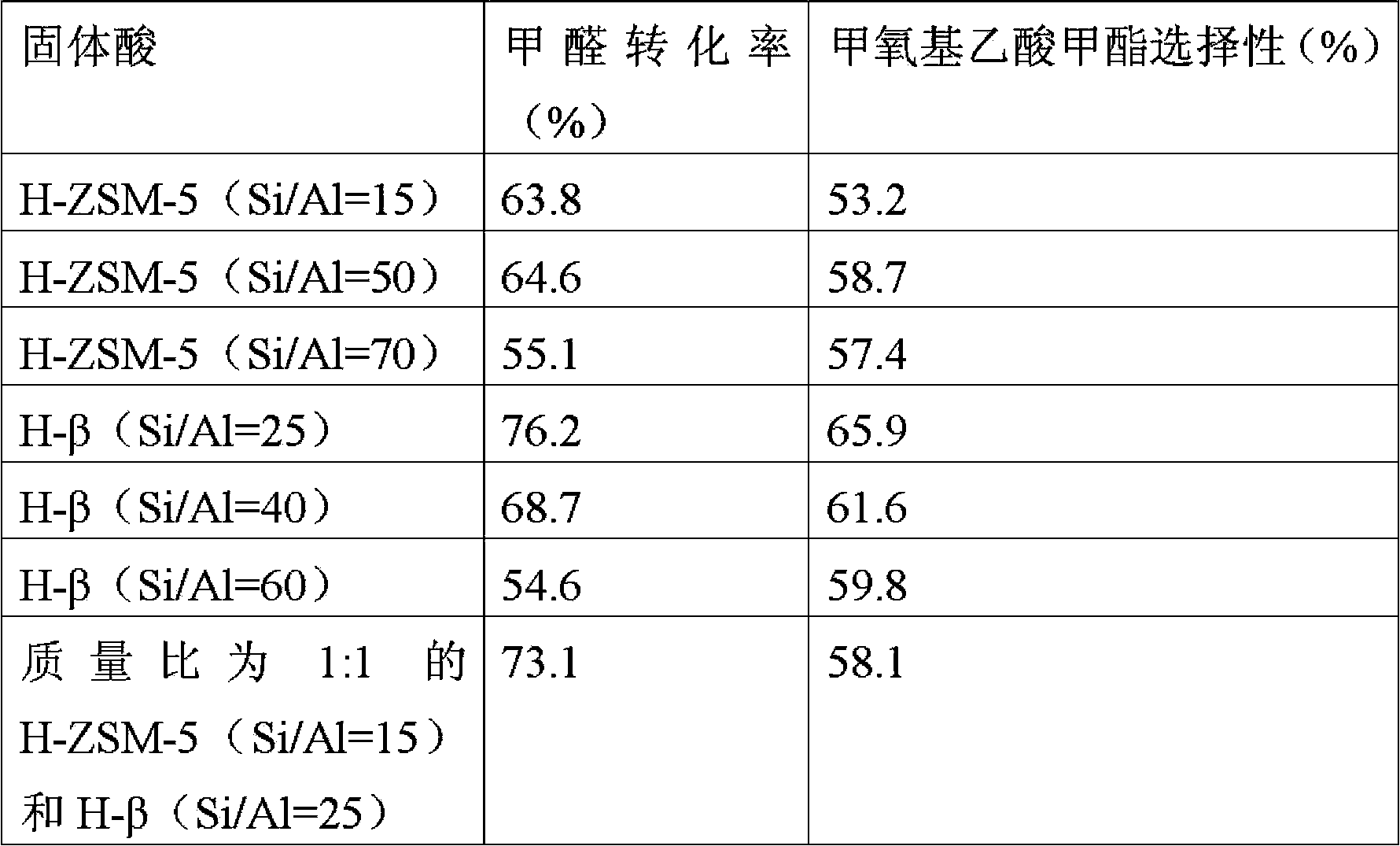
[0025] Other conditions were the same as in Example 1, and different solid acid catalysts were evaluated respectively, and the reaction effluent was analyzed by online chromatography. The results are shown in Table 1.
[0026] Table 1
[0027]
Embodiment 3
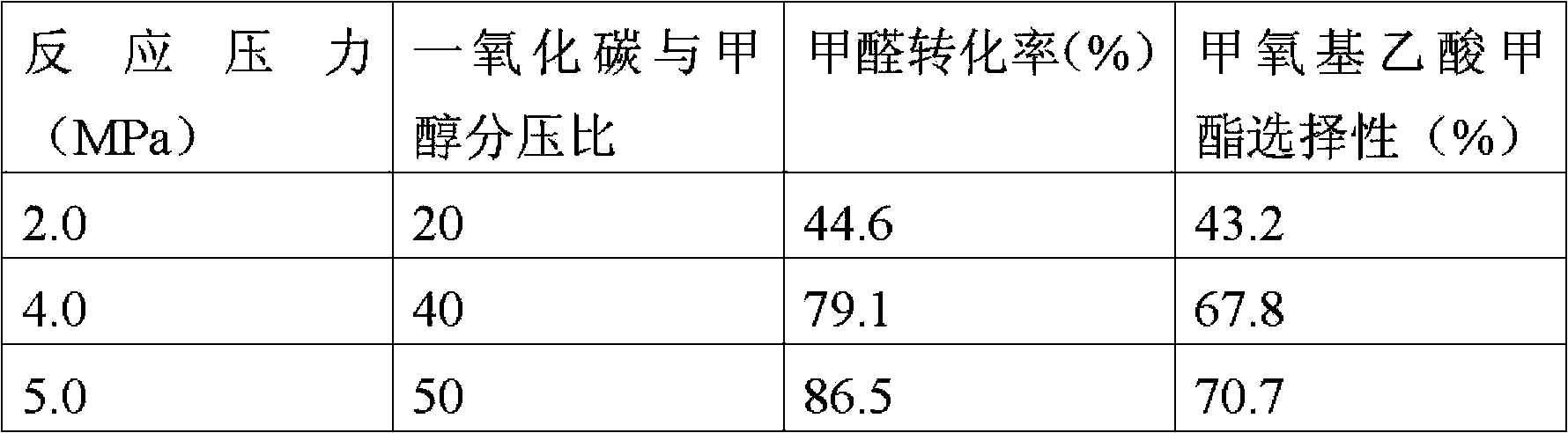
[0029] Other conditions were the same as in Example 1. The reaction performance of formaldehyde and methanol gas-phase carbonylation to produce methyl methoxyacetate was evaluated at different temperatures of 140°C, 150°C, and 170°C, and the reaction effluent was analyzed by online chromatography. The results are shown in Table 2.
[0030] Table 2
[0031] temperature(℃)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com