New compound having anthracycline antibiotic structure, and preparation method and application thereof
A technology of antibiotics and anthracyclines, which is applied in the field of new compounds containing anthracycline antibiotics structure and its preparation and application, can solve the problems of low solubility, decreased pharmacodynamic function of doxorubicin, and application limitations, etc., and achieve easy dissolution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
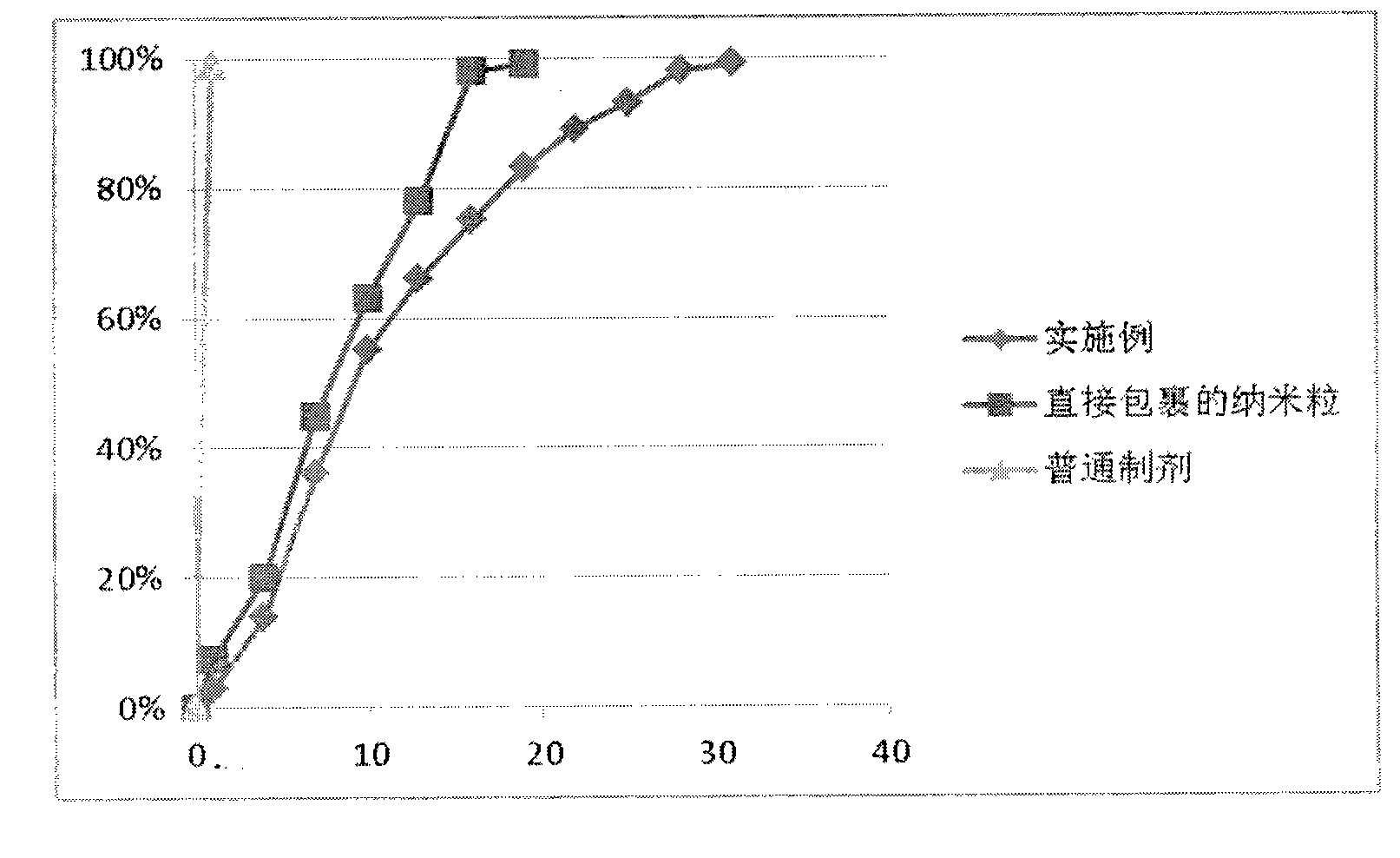
Examples
Embodiment 1
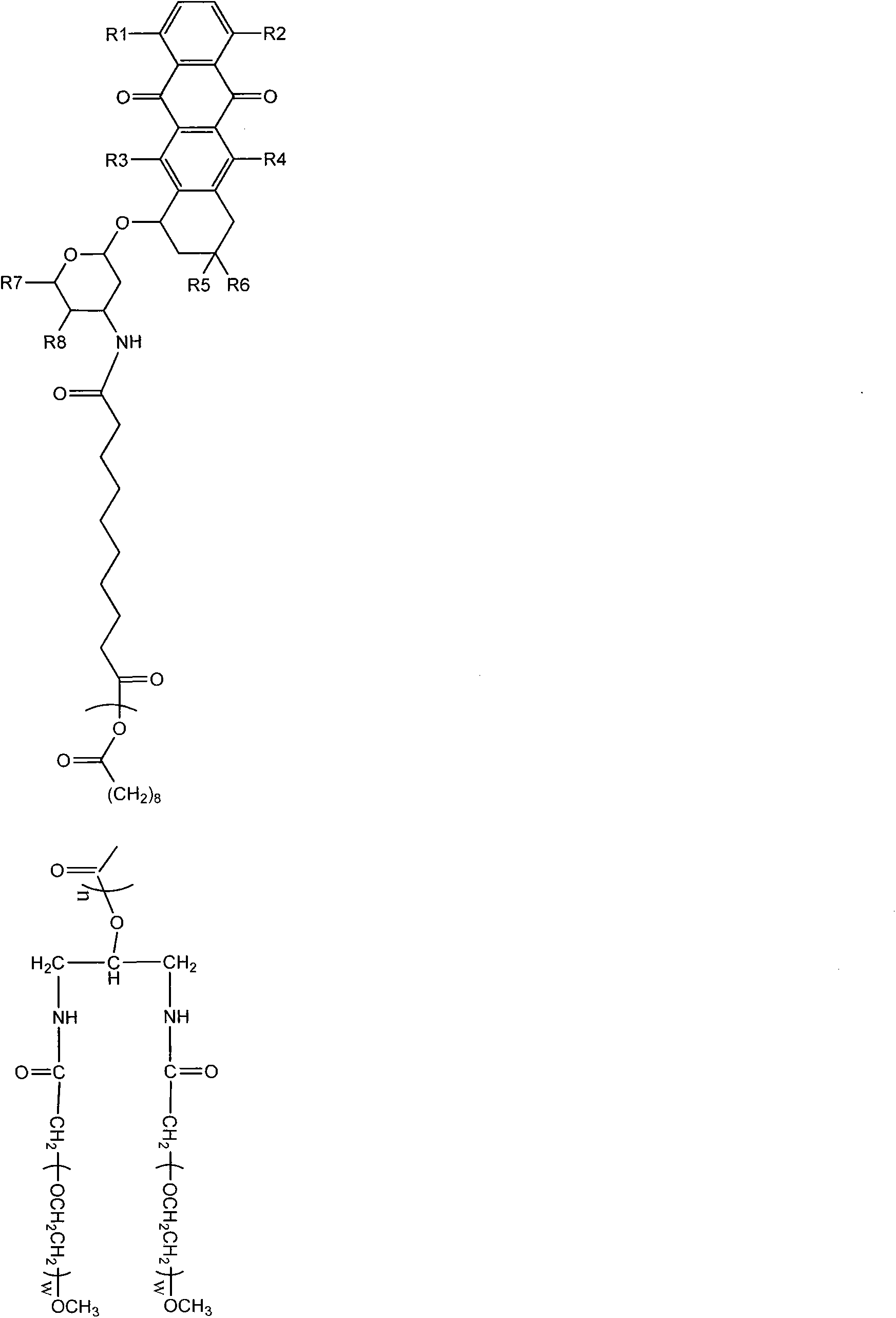
[0051] 1) A mixture of 2 g of sebacic acid in 25 ml of acetic anhydride is refluxed to form acetyl-sebacic acid;
[0052] 2) 5 g of compound A, 60 mg of compound B, 200 mg of dicyclohexylcarbodiimide and 10 mg of pyridine were mixed with 30 ml of dichloromethane, stirred at room temperature overnight; then washed with ether and dried under vacuum to obtain a polymer;
[0053] 3) Mix the products of step 1) and step 2) into a flask, and react at 150°C for 1 hour; the polymer is cooled to room temperature and dissolved in chloroform, washed with petroleum ether and dried;
[0054] 4) Put doxorubicin and the polymer of step 3) into a solution mixed with 10ml of dichloromethane and 6ml of dimethyl sulfoxide; then put it in an oven for 2 hours; minutes; the product was put into 5% cholic acid solution and stirred at 400 rpm for 2 hours; it was collected by centrifugation and freeze-dried to obtain the final product.
Embodiment 2
[0056] 1. Mix 2.5g of compound A, 47mg of compound B, 200mg of dicyclohexylcarbodiimide and 8mg of pyridine, add 20ml of dichloromethane, and stir overnight at room temperature;
[0057] It was then washed with ether and dried under vacuum;
[0058] 2. Mix 1 g of acetyl-sebacic acid (commercially available) and the product of step 1 into a flask, and react at 120°C for 1.5 hours; the polymer is cooled to room temperature and dissolved in chloroform, washed with petroleum ether and dried to obtain a polymer thing;
[0059] 3 Put pirarubicin and the polymer into a solution mixed with 5ml of methanol and 5ml of dichloromethane; then put it in an oven for 4 hours; at minus 10-20 degrees, ultrasonic for 20 minutes; put the product in 3% Stir in the cholic acid solution at 400 rpm for 2 hours; collect by centrifugation and freeze-dry to obtain the final product.
Embodiment 3
[0061] A mixture of 2 g of sebacic acid in 20 ml of acetic anhydride was refluxed to form acetyl-sebacic acid;
[0062] 2 Compound A 2.5g, Compound B 42mg, dicyclohexylcarbodiimide 150mg and tetrahydrofuran were mixed with 15ml of chloroform, stirred at room temperature overnight; then washed with ether and dried under vacuum to obtain a polymer;
[0063] 3 Mix the products of step 1 and step 2 into a flask, and react at 150°C for 1 hour; when the polymer is cooled to room temperature, dissolve it in chloroform, wash with petroleum ether and dry;
[0064] 4 Put arubicin and the polymer of step 3 into a solution mixed with 10ml of dichloromethane and 6ml of dimethyl sulfoxide; then put it in an oven for 8 hours; at minus 20-30 degrees, ultrasonic for 20 minutes The product was put into 5% cholic acid solution and stirred at 400 rpm for 2 hours; it was collected by centrifugation and freeze-dried to obtain the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com