Method for efficiently loading medicines in cell membrane microcapsules
A cell membrane, medium and high-efficiency technology, which is applied in the direction of pharmaceutical formulas, medical preparations of non-active ingredients, inorganic non-active ingredients, etc., can solve the problems of poor permeability of the capsule wall and only specific molecules can be loaded, and increase the permeability , Superior biocompatibility and transfer performance, and the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Add 20 μL of cytochalasin b solution (concentration: 1 mg / mL) to the culture dish of human vascular endothelial cells, incubate at 37°C for 30 minutes, wash with phosphate buffer three times, digest with trypsin, and collect cells by centrifugation; The obtained cells were vortexed for 1 min; the supernatant collected by centrifugation was the cell membrane microcapsule suspension.
[0022] Disperse 200 μg of the cell membrane microcapsules obtained above in 1 mL of phosphate buffer; add 10 μL of 1 mg / mL cell membrane red dye DiI to it, mix well, and incubate for 15 min; centrifuge and wash the obtained cell membrane microcapsules several times to remove unlabeled The dye on the membrane; see the fluorescent photo of the cell membrane microvesicles dispersed in phosphate buffered saline figure 1 .
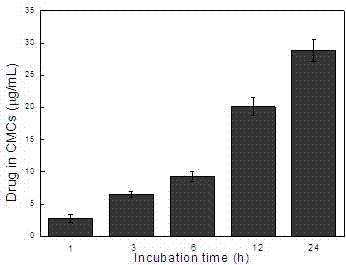
[0023] 2) Add 200 μg of the cell membrane microcapsules obtained in step 1) into the mixed solution of digitonin and doxorubicin hydrochloride, so that the concentration...
Embodiment 2
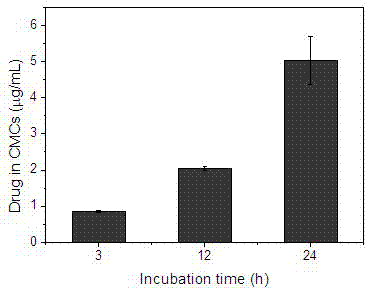
[0025] Same as Example 1, the difference lies in step 2), take 200 μg of the cell membrane microcapsules obtained in step 1) and add them to the mixed solution of digitonin and gadopentetate dimeglumine, so that the concentrations of digitonin and gadopentetate dimeglumine 10μg / mL and 200μg / mL respectively, incubate at 37°C for 3~24h; add calcium chloride solution to make the calcium ion concentration 5mM, incubate at 37°C for 2h; centrifuge and wash with phosphate buffer three times to obtain gadopentetate-loaded Cell membrane microcapsules of meglumine. According to inductively coupled plasma mass spectrometry, the drug concentration in the microcapsules is 0.8, 2, 16.2 μg / mL, see image 3 .
Embodiment 3
[0027] Same as Example 1, the difference lies in step 2), take 200 μg of the cell membrane microcapsules obtained in step 1) and add them to the mixed solution of digitonin and fluorescein-labeled dextran (molecular weight 4kD), so that digitonin and glucosinolate The concentrations of glycans were 10 μg / mL and 200 μg / mL, and incubated at 37°C for 3-24h; calcium chloride solution was added to make the calcium ion concentration 5mM, and incubated at 37°C for 2h; centrifuged and washed with phosphate buffer three times to obtain Cell membrane microcapsules loaded with dextran. According to the peak intensity of excitation at 488nm and emission at 525nm in the fluorescence spectrogram, the concentrations of fluorescein-modified dextran in the microcapsules are respectively 0.8, 0.9, and 1.1 μg / mL, see Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com