Terpyridyl derivative and application thereof to white light organic electroluminescent diode
A technology of terpyridine and its derivatives, which is applied in the direction of luminescent materials, organic chemistry, circuits, etc., and can solve problems other than the luminescent mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
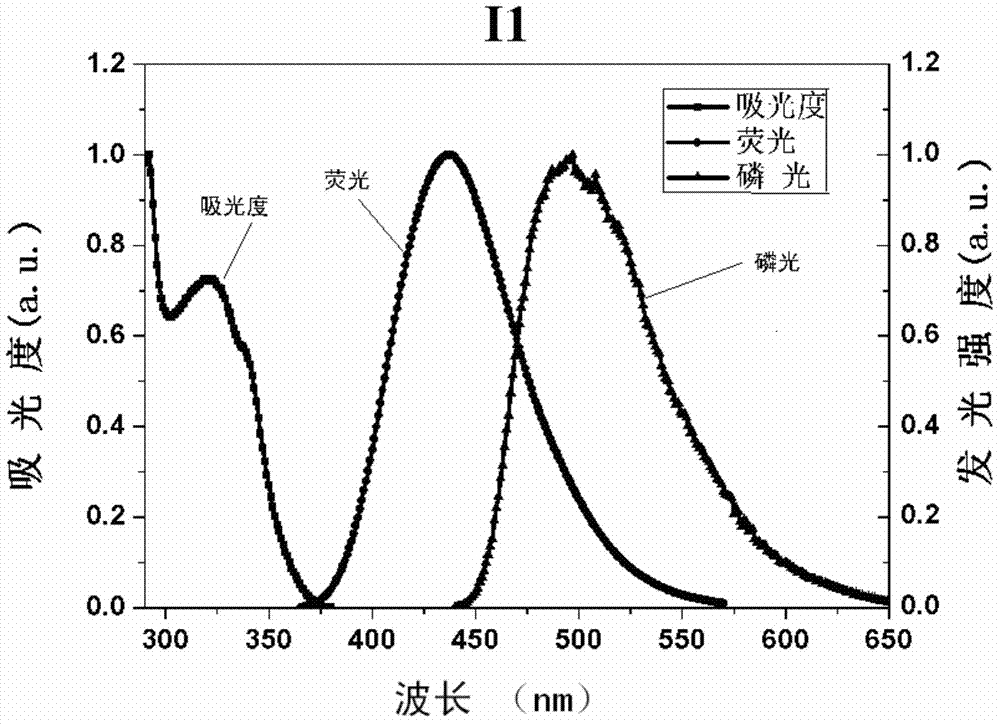
[0083] Synthesis of compound I1: the synthetic route is as follows:
[0084]
[0085] Synthesis of intermediate M1: Dissolve 3.7g of p-bromobenzaldehyde in 100ml of methanol solution, then add 0.8g of NaOH into methanol, and after it is completely dissolved, add 4.425ml of 40mmol 4-acetylpyridine dropwise to the methanol solution, at room temperature After 24 hours of reaction, the reaction solution was extracted with chloroform, washed with water and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent was dichloromethane:methanol=20:1v / v. After vacuum drying, 4.8g of white solid M1 of 62% was obtained.
[0086] Synthesis of compound I1: 0.7gM1, 0.57g4-(9H-9-carbazolyl)phenylboronic acid, 0.231g, tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 ), 6mL of 2M sodium carbonate, 18mL of toluene and 9mL of absolute ethanol were heated and s...
Embodiment 2
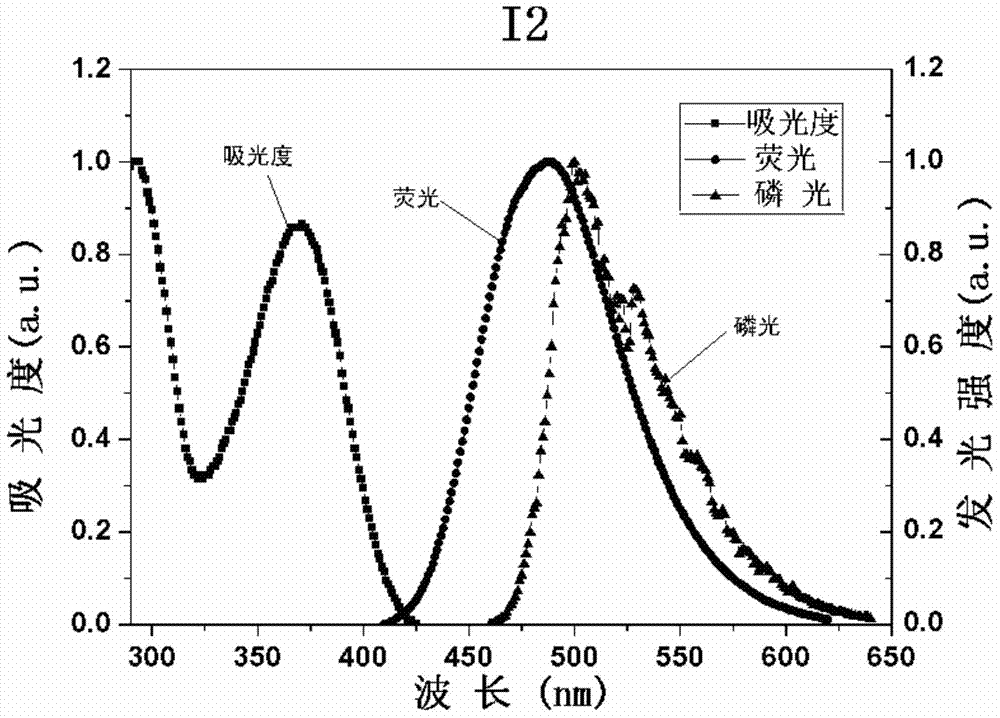
[0090] The synthetic route of compound I2 is as follows:
[0091]
[0092] The synthesis of intermediate M1 is the same as in Example 1.
[0093] Synthesis of compound I2: 0.7g M1, 0.65g bis(4-tert-butylphenyl)amine, 0.021g bis(dibenzylideneacetone) palladium (Pd(dba)2) (Is the Chinese correct?) g2-di Cyclohexylphosphonium-2',4',6'-triisopropylbiphenyl (x-phos), 0.7g sodium tert-butoxide (tBuONa) and 30mL toluene were heated and stirred at 90°C for 12h under nitrogen protection. After the reaction solution was extracted with chloroform, washed with water and dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent was dichloromethane:methanol=20:1v / v. After vacuum drying, 0.846g of white solid I2 was obtained with a yield of 80%.
[0094] Product MS (m / e): 588, corresponding to: C41H40N4=588, proving that the compound is I2. 1H NMR (400MHz, CDCl3...
Embodiment 3
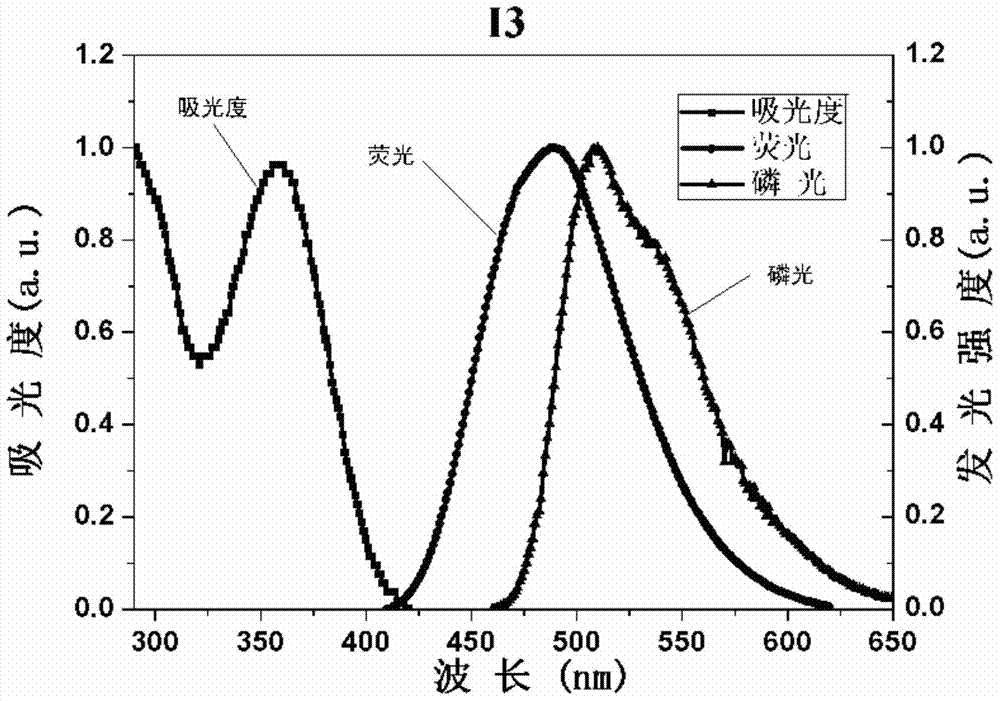
[0097] The synthetic route of compound I3 is as follows:
[0098]
[0099]The synthesis of intermediate M1 is the same as in Example 1.
[0100] The synthesis of compound I3: the M1 of 0.7g, 0.58g4-boric acid triphenylamine), 0.231g tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 ) 2M sodium carbonate, 18mL toluene and 9mL absolute ethanol were heated and stirred at 90°C for 12h under nitrogen protection. After the reaction solution was extracted with chloroform, washed with water and dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent was dichloromethane:methanol=20:1v / v. After vacuum drying, 0.835g of white solid I3 was obtained with a yield of 84%.
[0101] Product MS (m / e): 552, corresponding to: C39H28N4=552, proving that the compound is I3. 1H NMR (400MHz, CDCl3) δ8.83 (s, 4H), 8.20 (d, J=5.2Hz, 4H) ,8.14(s,2H),7.80(dd,J=20.6,8.4H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com