Folic acid-benzaldehyde nitrogen mustard-HPMA macromolecule copolymer and preparation and application thereof
A polymer copolymer and technology of benzaldehyde nitrogen mustard, applied in the field of polymer chemistry, can solve problems such as inconvenient administration, poor water solubility of benzaldehyde nitrogen mustard, and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) compound Preparation: Weigh folic acid (441.4 mg, 1.0 mmol), cyclohexylcarbodiimide (247.6 mg, 1.2 mmol) and N-hydroxysuccinimide (230.2 mg, 2.0 mmol), dissolve in 30 mL of di In methyl sulfoxide, react in the dark at 45~55°C for 6~8h. After all the folic acid is converted into folic acid activated ester, add 571.0 mg (10 mmol) of allylamine to the reaction solution, and keep it in the dark at -5~5°C. Light reaction 12~15h. Add acetone to precipitate, filter with suction, and dry in a vacuum oven to obtain the compound (321.2 mg, yield 65%).
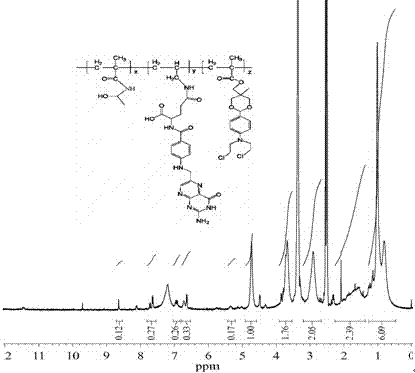
[0046] 1 H NMR (400MHz, CDCl3): δ 1.93 (m, 2H, -CH(COOH)C H 2 CH 2 CO-), 2.23 (t, 2H, -CH(COOH)CH 2 C H 2 CO-), 3.42 (s, 2H, -NHC H 2 CH=CH 2 ), 4.20 (s, 2H, -NHC H 2 -Heterocyclic), 4.46 (t, 1H, -C H (COOH)CH 2 CH 2 CO-), 5.22 (dd, 2H, -NHCH 2 CH=C H 2 ), 5.89 (m, 1H, -NHCH 2 C H =CH 2 ), 6.63 (d, 2H, -NHC 6 H 2 h 2 CO-), 7.62 (d, 2H, -NHC 6 h 2 H 2 CO-), 8.6 (s, 1H, Heterocyclic).
[0047] (...
Embodiment 2
[0054] compound , , Preparation: with embodiment 1.
[0055] Preparation of copolymer: Weigh HPMA (0.218 g, 1.52 mmol, 90 mol%) into a Shleck bottle, heat to dissolve with 0.5 mL of DMSO, then add 0.5 mL of acetone; weigh the compound (0.044 g, 0.09 mol, 5 mol%) and compound (0.040 g, 0.09 mmol, 5 mol%) was added to the Shleck bottle, stirred until dissolved, and when it was cooled to room temperature, 0.024 g (8%, wt) azoisobutyronitrile (AIBN) was added, vacuumized and nitrogen cycled 3~5 times, keep the temperature at about 55°C after sealing, and react for 24 hours. Precipitate with a mixture of acetone and ether (volume ratio 7:3), filter and dissolve the precipitate with 1 mL of anhydrous methanol, centrifuge with an ultrafiltration concentration centrifuge tube with a molecular weight of 3000, remove small molecules, and obtain 0.22 g of polymer , yield 73%.
[0056] m n =2.4×10 4 , M w / M n =1.56. 1 H-NMR (400 MHz, DMSO, δ, ppm): 8.6 (Heterocyclic of ...
Embodiment 3
[0058] compound , , Preparation: with embodiment 1.
[0059] Preparation of copolymer: Weigh HPMA (0.218 g, 1.52 mmol, 84 mol%) into a Shleck bottle, heat to dissolve with 0.5 ml of DMSO, then add 0.5 mL of acetone; weigh the compound (0.066 g, 0.14 mmol, 8%) and compound (0.056 g, 0.14 mmol, 8%) was added to the Shleck bottle, stirred until dissolved, and when it was cooled to room temperature, 0.034 g (10%, wt) azoisobutyronitrile (AIBN) was added, vacuumized and nitrogen cycled for 3 ~5 times, keep the temperature at about 55°C after sealing, and react for 24 hours. Precipitate with a mixture of acetone and ether (volume ratio 7:3), filter and dissolve the precipitate with 1 mL of anhydrous methanol, centrifuge with an ultrafiltration concentration centrifuge tube with a molecular weight of 3000, remove small molecules, and obtain 0.246 g of polymer , yield 72%.
[0060] m n =2.6×10 4 , M w / M n =1.23. 1 H-NMR (400 MHz, DMSO, δ, ppm): 8.6 (Heterocyclic of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com