Adamantyl-modified near-infrared squaraine dye as well as preparation method and application thereof
An adamantyl and infrared square technology is applied in the field of adamantyl-modified near-infrared squaraine dyes and their preparation, which can solve the problems of limited application, easy aggregation, fading and the like, and achieves improved solubility, excellent optical properties, and stability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
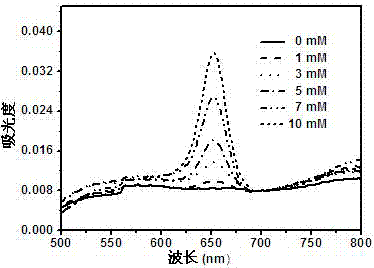
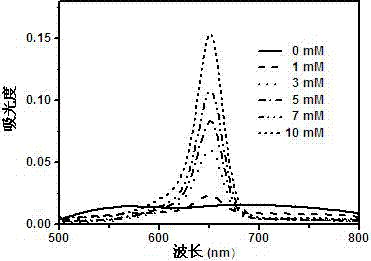
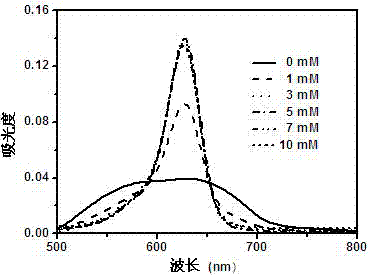
Image
Examples
Embodiment 1
[0051] 1-Adamantyl-modified amide derivatives , n = 0 preparation:
[0052] In a 50 mL round bottom flask, add 1-adamantanecarboxylic acid (1.00 g, 5.55 mmol), 5 mL of thionyl chloride, at 85 ° C was refluxed for 1 h. Cool to room temperature after the reaction, and distill off excess thionyl chloride under reduced pressure. Add 5 mL of dry toluene to the above-mentioned reaction flask, add 10 mL of a toluene solution of N-methylaniline (1.00 mL, 9.23 mmol) dropwise at room temperature, and complete the dropwise addition in about 2 h, and continue stirring for 1 h after the dropwise addition. After the reaction stopped, washed several times with water, dried and removed the solvent under reduced pressure, then separated by silica gel column chromatography, the developing solvent was petroleum ether:ethyl acetate (v / v)=50:1, and 1.20 g of white solid was obtained with a yield of 80 %. Melting point: 104-105℃; IR (KBr): 3059 (Ar-H), 2901, 2850 (C-H), 1628 (C=O) cm -1 ; 1 ...
Embodiment 2
[0054] 1-Adamantyl-modified amide derivatives , n = 1 preparation:
[0055] In a 50 mL reaction flask, add 1-adamantaneacetic acid (1.00 g, 5.15 mmol), 5 mL thionyl chloride, and reflux for 3 h. After the reaction stopped, it was cooled, and excess thionyl chloride was removed under reduced pressure, and the obtained product was directly used in the next reaction. Add 10 mL of dry toluene to the above-mentioned reaction flask, add 10 mL of a toluene solution of N-methylaniline (1.50 mL, 13.8 mmol) dropwise at room temperature, and complete the dropwise addition in about 2 h, and continue stirring for 3 h after the dropwise completion. After removing the solvent under reduced pressure, separate by silica gel column chromatography, gradient elution, first rinse with petroleum ether: ethyl acetate (v / v) = 100:1, remove excess N-methylaniline, and then use petroleum ether: Ethyl acetate (v / v)=30:1 was used as developing solvent to elute, and 1.43 g of white solid was obtained w...
Embodiment 3
[0057] 1-adamantyl modified aniline derivatives , n = 0 preparation:
[0058] In a 50 mL three-neck flask, add 1-adamantyl-modified amide derivatives, n = 0 (0.54 g, 2.00 mmol), NaBH 4 (0.18 g, 4.76 mmol) and 10 mL of dry THF, cooled to 0 ° c. then in N 2 Under protection, drop 20 mL dissolved in I 2 (1.00 g, 3.94 mmol) in THF, drop it within 2.5 h. Rise to 70 after dripping ° C, continue to react for 36 h. After the reaction was completed, it was cooled to room temperature, and a small amount of water was added to quench the reaction. After removing most of the solvent under reduced pressure, add 30 mL of ether, wash with water, anhydrous MgSO 4dry. After the solvent was removed under reduced pressure, it was separated by silica gel column chromatography with petroleum ether as the eluent to obtain 80 mg of a colorless viscous liquid with a yield of 16%. IR (KBr): 2895, 2883, 2844, 2817, 1610, 1597, 1506, 1448, 1360, 1236, 1182, 743, 689 cm -1 ; 1 H NMR (400 MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com