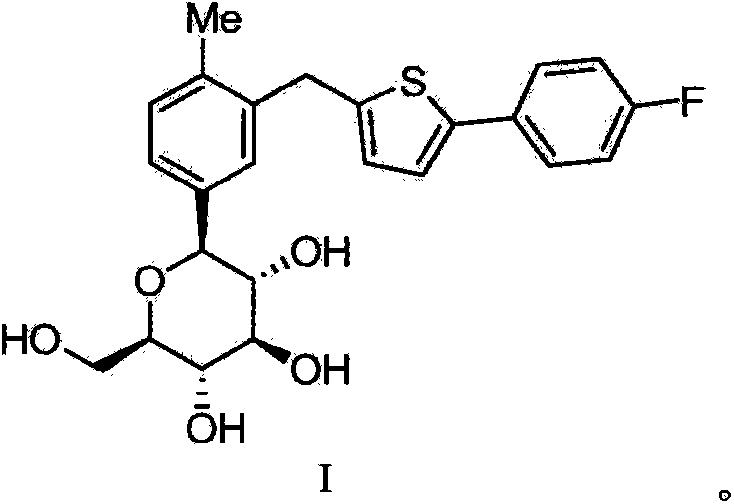
High-purity canagliflozin compound and preparation method thereof
A technology of compounds and complexes, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0066]
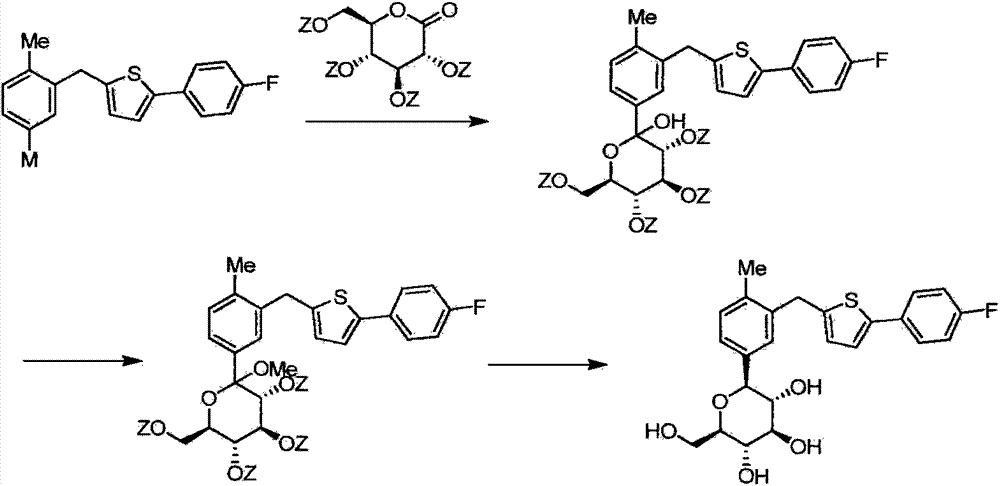
[0067] According to the method in Journal of Medicinal Chemistry2010,53,6355-60, trimethylsilyl protected canagliflozin (formula 1) was obtained, and then trimethylsilyl and methoxy were removed according to the method published in the document, Canagliflozin (formula 2) was obtained. The specific method is as follows:
[0068] Compound 1 (6.3kg, 13.2mol) was added to 60L of dichloromethane, triethylsilane (4.6kg, 39.9mol) was added and cooled with dry ice acetone, boron trifluoride ether solution (5L, 39.5mmol) was added dropwise Then, the reaction mixture was heated to 0° C., stirred for 2 hours, and saturated aqueous sodium bicarbonate (80 L) was slowly added to the reaction kettle to quench the reaction. Separate the layers, concentrate the organic phase under reduced pressure to dryness, add 100L of water and 70L of ethyl acetate, stir and extract, then extract the water phase with 70L of ethyl acetate, combine the organic phases, wash with 50L of water, and ...
Embodiment 1
[0081] Canagliflozin (10.0g, 22.5mmol) was dissolved in absolute ethanol (200ml), added L-proline (7.8g, 67.7mmol, 3eq), heated to reflux, all solids were dissolved, and then slowly Cool to room temperature, filter the precipitated solid, and wash the filter cake with absolute ethanol to obtain canagliflozin L-proline complex.
[0082] Add the complex to purified water (200ml), adjust the pH to 2 with hydrochloric acid, extract with methyl tert-butyl ether (100ml×3), combine the organic phases, wash with saturated sodium chloride solution, concentrate, and a large amount of solid Precipitated, filtered, and the filter cake was washed with a small amount of cold methyl tert-butyl ether, and vacuum-dried at 50°C to obtain 8.9 g of canagliflozin finished product, with a yield of 89%.
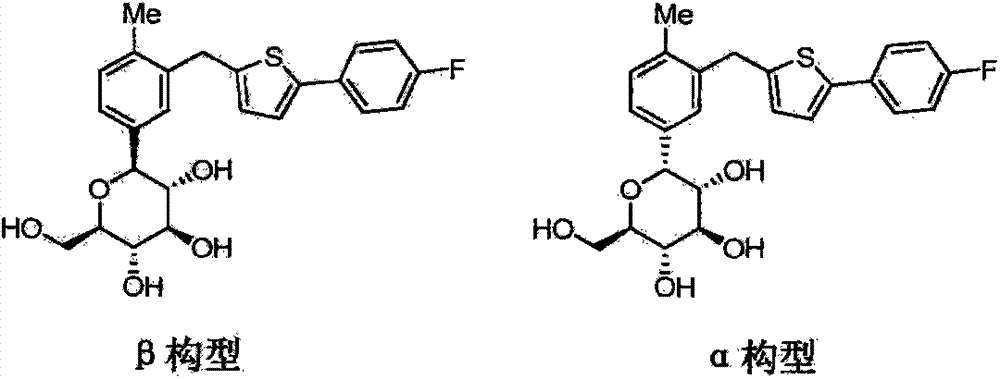
[0083] HPLC detection of α-configuration impurity: 0.017%.
Embodiment 2
[0085] Canagliflozin (17.2g, 38.7mmol) was added to 95% ethanol (250ml), L-proline (11.1g, 96.4mmol, 2.5eq) was added, heated to reflux, all the solids were dissolved, and then slowly Slowly cooled to 10°C, gradually a solid precipitated out. The precipitated solid was filtered, and the filter cake was washed with absolute ethanol to obtain the canagliflozin L-proline complex.
[0086] Add the complex to purified water (200ml), adjust the pH to 1 with hydrochloric acid, extract with ether (150ml×3), combine the organic phases, wash with saturated sodium chloride solution, concentrate, a large amount of solids precipitate out, filter, filter The cake was washed with a small amount of cold diethyl ether and dried under vacuum at 55°C to obtain 15.4 g of canagliflozin finished product with a yield of 90%.
[0087] HPLC detection of α-configuration impurity: 0.026%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com