A kind of alkyl substituted dendritic metalloporphyrin and its preparation method and application
A dendritic metal porphyrin technology, applied in the field of alkyl substituted dendritic metal porphyrin and its preparation, achieves the effects of easy operation, increased yield, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
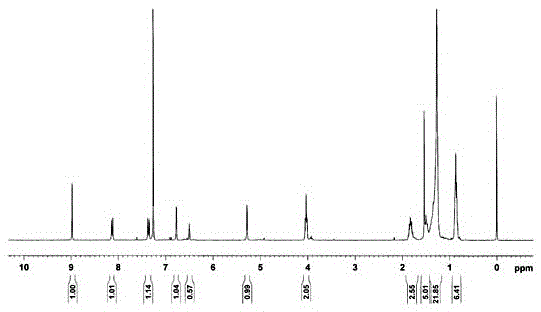
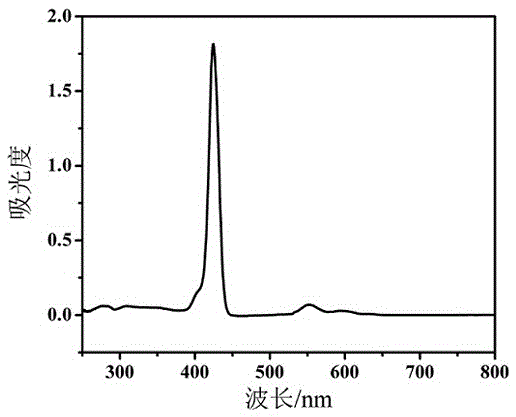
[0027] G 2 - Preparation of ZnPor:
[0028] Dissolve 37mg (ie 0.050mol) of 5,10,15,20-p-hydroxyphenyl zinc porphyrin in 10ml of dry DMF, then add 35mg (ie 0.25mol) of sodium carbonate, and stir for 5min in a nitrogen atmosphere; 130mg (ie 0.24mol) [C 12 h 25 O] 8 G 1 -Br, 26mg (ie 0.10mol) of 18-crown-6 ether and 1mg (ie 0.006mol) of potassium iodide were stirred and reacted for 24h in a nitrogen atmosphere at a temperature of 80°C. Then, the reaction solution is post-treated. First add 20ml of distilled water to the reaction solution, stir, and let stand to separate layers; then remove the organic phase of the lower layer and extract it with 20ml of dichloromethane, repeat the extraction 3 times, combine the extracts and evaporate to dryness, and remove the solvent under reduced pressure; Chloromethane is used as eluent, purifies with silica gel column, obtains [C 12 h 25 O] 8 G 1 - Crude DPZn. Will [C 12 h 25 O] 8 G 1 -The crude product of DPZn is dissolved in...
Embodiment 2
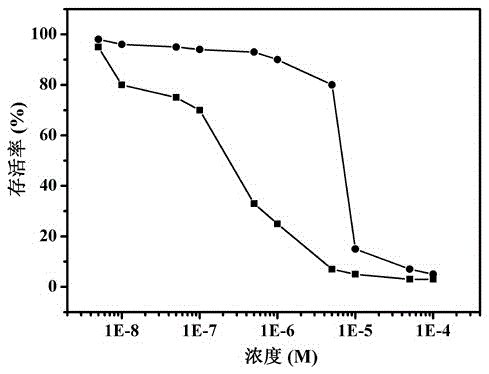
[0030] Photocytotoxicity evaluation of sensitizers:
[0031] To quantitatively evaluate the cytotoxicity of sensitizers, HeLa cells were treated with 6.25 μM [C 12 h 25 O] 8 G 1 -DPZn was incubated together to complete the determination of cell viability, while non-alkyl modified dendritic metalloporphyrin G 2 -DPZn as reference.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com