Asymmetric dendritic metalloporphyrin as well as preparation method and application thereof
A dendritic metal, asymmetric technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of complex preparation process, difficult reaction control, and the need to improve the effect of photodynamic therapy. Achieve the effect of simple process, mild conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] G 2 - Preparation of ZnPor:
[0054] Dissolve 28 mg (ie 0.038 mol) of 5-p-hydroxyphenyl-10, 15, 20-trichlorophenylporphyrin in 10 ml of chloroform and stir;
[0055] Dissolve 21 mg (0.114 mol) of zinc acetate in a mixture of 10 ml of chloroform and 20 ml of methanol to obtain a zinc acetate solution;
[0056] The zinc acetate solution is placed in the constant pressure funnel; the zinc acetate solution in the constant pressure funnel is added dropwise to the 5-p-hydroxyphenyl-10,15,20-trichlorophenylporphyrin solution; the dropping time is controlled About 15 minutes; after the dropwise addition, stir for 16 hours at a temperature of 25°C;
[0057] Take 30 ml of distilled water and add it to the reaction solution, stir and let it stand. After the layers are separated, the organic phase of the lower layer is dried with anhydrous sodium sulfate and evaporated to dryness to obtain 5-p-hydroxyphenyl-10, 15, 20-trichloro Crude phenyl zinc porphyrin;
[0058] Dissolv...
Embodiment 2
[0063] G 3 - Preparation of ZnPor:
[0064] Dissolve 30 mg (ie 0.038 mol) of 5-p-hydroxyphenyl-10, 15, 20-trichlorophenyl zinc porphyrin prepared in Example 1 and 33 mg (ie 0.041 mol) of [G-3]Br in 20 ml of dry acetone; then add 2.4 mg (ie 0.017 mol) of potassium carbonate and 9.5 mg (ie 0.036 mol) of 18-crown-6 ether as a catalyst; under the temperature condition of 56 ℃, in nitrogen atmosphere, stir the reaction for 60 h .
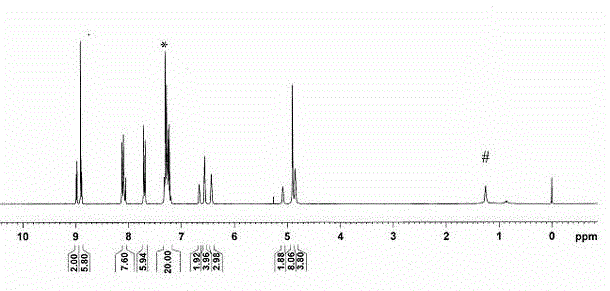
[0065] Then, the reaction solution is post-treated. First add 30 ml of distilled water to the reaction solution, stir, and let stand to separate layers; then take the lower organic phase and extract with 20 ml of dichloromethane, spin the extract to dryness, and remove the solvent under reduced pressure; then use dichloromethane as the eluent liquid, purified with a silica gel column to obtain G 3 -ZnPor Crude. Will G 3 - Dissolve the crude ZnPor in 20 ml of chloroform, carefully inject 120 ml of methanol, and let it stand, the precipitated crystal...
Embodiment 3
[0068] G 4 - Preparation of ZnPor:
[0069] Dissolve 30 mg (ie 0.038 mol) of 5-p-hydroxyphenyl-10, 15, 20-trichlorophenyl zinc porphyrin prepared in Example 1 and 77 mg (ie 0.044 mol) of [G-4]Br in 10 ml of dry 2.0 mg (ie 0.015 mol) of potassium carbonate and 9.0 mg (ie 0.035 mol) of 18-crown-6 ether were added as catalysts; at 56°C, in a nitrogen atmosphere, the reaction was stirred for 42 h.
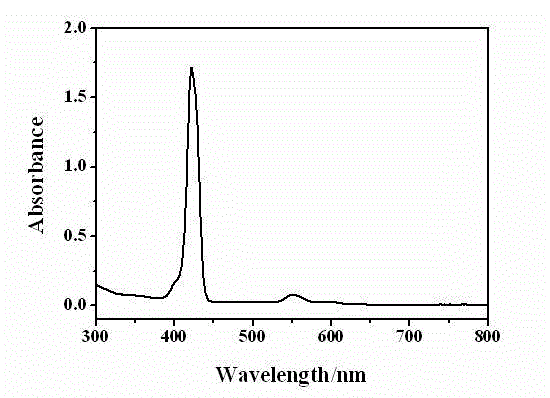
[0070] Then, the reaction solution is post-treated. First add 20 ml of distilled water to the reaction solution, stir, and let stand to separate layers; then take the lower organic phase and extract with 20 ml of dichloromethane, spin the extract to dryness, and remove the solvent under reduced pressure; then use dichloromethane as the eluent , Purify with silica gel column to get G 4 -ZnPor Crude. Will G 4 - Dissolve the crude ZnPor in 20 ml of chloroform, carefully inject 120 ml of methanol, and let it stand, the precipitated crystal is G 4 -ZnPor, yield 60%.
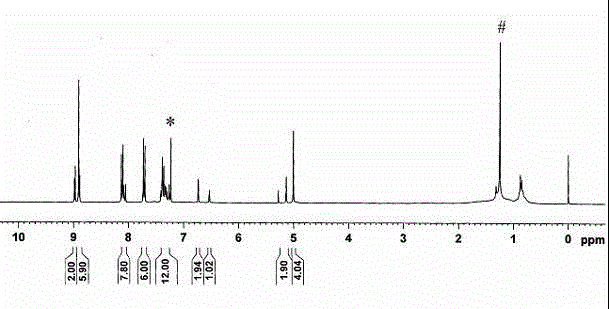
[0071] NMR spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com