Copolymer containing cyclopentadiene bithiophene-benzobis(benzothiadiazole), preparation method and applications thereof
A technology of unit naphthalene tetracarboxylic acid diimide copolymer and thiadiazole, applied in the field of cyclopentadiene dithiophene-benzobis(benzothiadiazole) copolymer and its preparation and application, It can solve the problems of low conversion efficiency and achieve the effects of simple structure, good electron delocalization performance and excellent charge transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
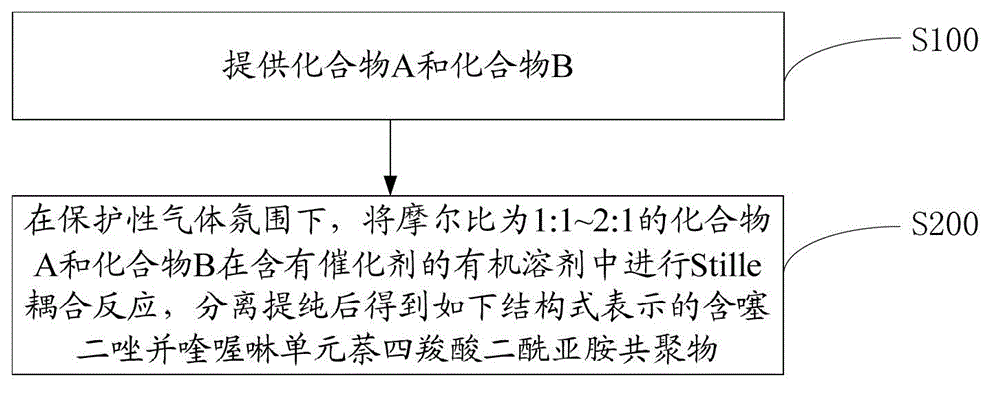
[0048] One embodiment contains the preparation method of thiadiazoloquinoxaline unit naphthalene tetracarboxylic acid diimide copolymer, such as figure 1 shown, including the following steps:
[0049] Step S100, providing compound A and compound B.
[0050] A is: B is: Among them, R 1 for C 1 ~C 20 The alkyl group or the following structural unit:
[0051] R 2 , R 3 , R 4 for H, C 1 ~C 20 Alkyl or C 1 ~C 20 The alkoxyl group, R 5 , R 6 , R 7 for H or C 1 ~C 20 of alkyl.
[0052] Wherein the preparation of compound A comprises the following steps:
[0053] Step S111, providing compound C and compound D represented by the following structural formula,
[0054] C is: D is: R 1 -NH 2 , where R 1 for C 1 ~C 20 The alkyl group or the following structural unit: R 2 , R 3 , R 4 for H, C 1 ~C 20 Alkyl or C 1 ~C 20 of alkoxy.
[0055] It should be noted that C 1 ~C 20 The alkyl group is C 1 ~C 20 branched chain alkyl or C 1 ~C 20 branched c...
Embodiment 1
[0088] This embodiment discloses poly N,N'-di-methyl-1,4,5,8-naphthalene tetracarboxylic acid diimide-6,7-dihexyl-4,9-di(4 -Ethylthiophen-2-yl)[1,2,5]thiadiazolo[3,4-g]quinoxaline (copolymer of naphthalene tetracarboxylic diimide containing thiadiazoloquinoxaline unit P1):
[0089]
[0090] The preparation process of the above-mentioned naphthalene tetracarboxylic acid diimide copolymer P1 containing thiadiazoloquinoxaline units is as follows:
[0091] 1.1. Preparation of N,N'-dimethyl-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide (compound A)
[0092]
[0093] Compound C and compound D are provided, compound C is 2,6-dibromo-1,4,5,8-naphthalene dianhydride, and compound D is n-octylamine (C 8 h 17 NH 2 );
[0094] Under nitrogen protection, methylamine (compound D) (1mmol) was added to a solution of propionic acid (15mL) containing 2,6-dibromo-1,4,5,8-naphthalene dianhydride (0.1mmol), and reflux 12 hours. After cooling to room temperature, the rea...
Embodiment 2
[0106] This example discloses poly N,N'-bis-(1-octylnonyl)-1,4,5,8-naphthalene tetracarboxylic acid imide-6,7-bis(1-octylnonyl) with the following structural formula Alkyl)-4,9-di(thiophen-2-yl)[1,2,5]thiadiazolo[3,4-g]quinoxaline (containing thiadiazoloquinoxaline unit naphthalene tetracarboxylic Acid diimide copolymer P2):
[0107]
[0108] The preparation process of the above-mentioned naphthalene tetracarboxylic acid diimide copolymer P2 containing thiadiazoloquinoxaline units is as follows:
[0109] 2.1. Preparation of N,N'-bis-(1-octylnonyl)-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide (compound A)
[0110]
[0111] Provide compound C and compound D, compound C is 2,6-dibromo-1,4,5,8-naphthalene dianhydride, compound D is 1-octyl-nonylamine;
[0112] Under nitrogen protection, 1-octyl-nonylamine (compound D) (0.255g) was added to 2,6-dibromo-1,4,5,8-naphthalene dianhydride (compound C) (0.1mmol ) in propionic acid (15 mL) and reflux for 14 hours....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com