Drug nanoparticle preparation based on complexing coating and preparation method and application
A technology of nanoparticles and complexes, which is applied in the field of drug nanoparticle preparations based on complex coating, can solve the problems of difficult promotion and high cost, and achieve controllable size and shape, low cost, good shape and Effects with easy size control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] It is the ethanol solution of paclitaxel that volume mass concentration is 40mg / mL, the aqueous solution of tannic acid that volume mass concentration is 80mg / mL and the FeCl that volume mass concentration is 20mg / mL 2 Under ultrasonic conditions, the ethanol solution of the above-mentioned paclitaxel of 1ml is added to 100ml water, obtains the dispersion system that contains paclitaxel particle; In the above-mentioned dispersion system, add the tannic acid aqueous solution of 1ml respectively (concentration is 80mg / mL ) and 1ml volume mass concentration is 20mg / mL FeCl 2 aqueous solution; and after continuous ultrasonication for 30 seconds, a stable paclitaxel nanoparticle solution preparation was obtained; the suspension was centrifuged to obtain paclitaxel nanoparticle powder.
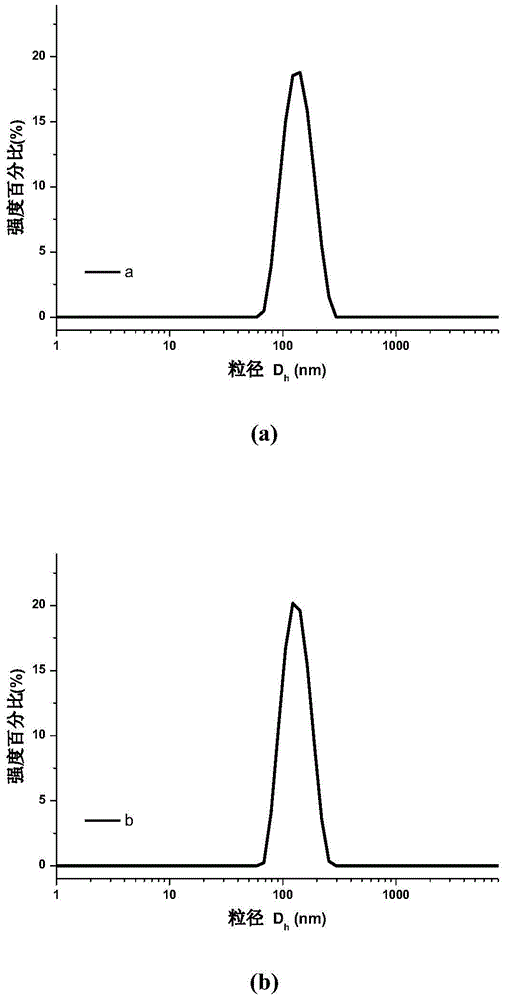
[0046] The particle size distribution of the obtained nanoparticles is shown in the attached figure 1 (a); The measured Zeta potential of the nanoparticles is -21.7eV (attached figure 2 In...
Embodiment 2
[0048] Configure the glycerin solution of paclitaxel with a volume mass concentration of 2 mg / mL, the aqueous solution of gallic acid with a volume mass concentration of 200 mg / mL and the CuSO with a volume mass concentration of 2 mg / mL 4 Under ultrasonic conditions, the glycerin solution of the above-mentioned paclitaxel of 10ml is added in 100ml water, obtains the dispersion system that contains paclitaxel particle; Rapidly in above-mentioned dispersion system, add the gallic acid aqueous solution of 1ml respectively (concentration is 200mg / mL) And 1ml volume mass concentration is 2mg / mL CuSO 4 aqueous solution; and after continuous ultrasonication for 5 seconds, a stable paclitaxel nanoparticle solution formulation is obtained;
[0049] The particle size distribution of the obtained nanoparticles is shown in the attached figure 1 (b); The measured Zeta potential of the nanoparticles is -24.3eV (attached figure 2 in b). Scanning electron microscope pictures of the obtain...
Embodiment 3
[0051] It is the ethanol solution of the docetaxel that volume mass concentration is 2mg / mL, the aqueous solution of the catechol of 80mg / mL volume mass concentration and the aqueous solution of the zinc oxalate that volume mass concentration is 20mg / mL; The ethanol solution of the above-mentioned docetaxel was added to 100ml water to obtain a dispersion system containing docetaxel particles; quickly to the dispersion system, add 1ml of catechol aqueous solution (concentration is 80mg / mL) and 1ml volume mass concentration It is an aqueous solution of 20 mg / mL zinc oxalate; and after continuous ultrasonication for 30 seconds, a stable docetaxel nanoparticle solution preparation is obtained;
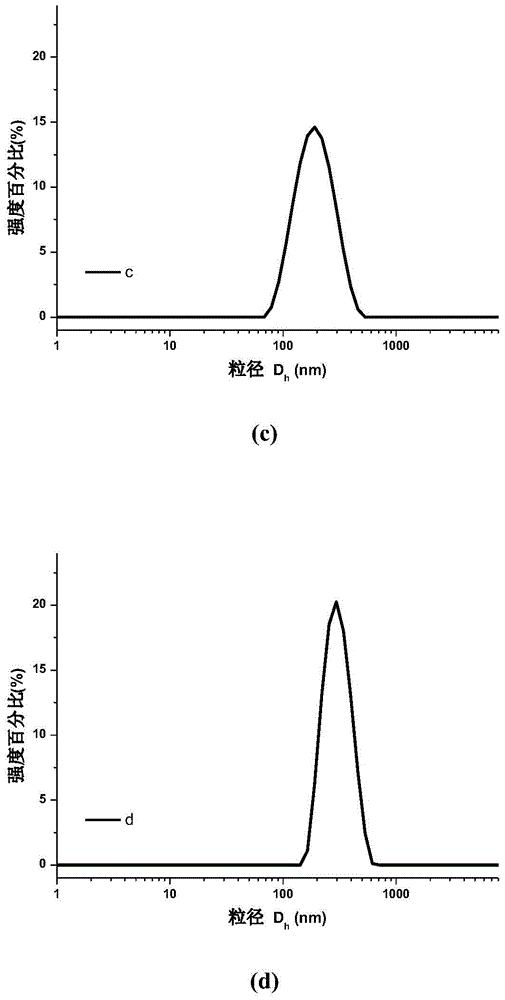
[0052] The particle size distribution of the obtained nanoparticles is shown in the attached figure 1 (c).
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com