Composite film for guiding bone tissue regeneration and preparation method of composite film
A bone tissue regeneration and composite technology, applied in medical science, surgery, etc., can solve the problem of unsatisfactory mechanical properties and degradation properties of PCL, unfavorable deposition of bone-like apatite, cell adhesion, and further improvement in degradation properties, etc. problems, to achieve the effect of being conducive to surgical operation, good physical barrier effect, and long-term space maintenance ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
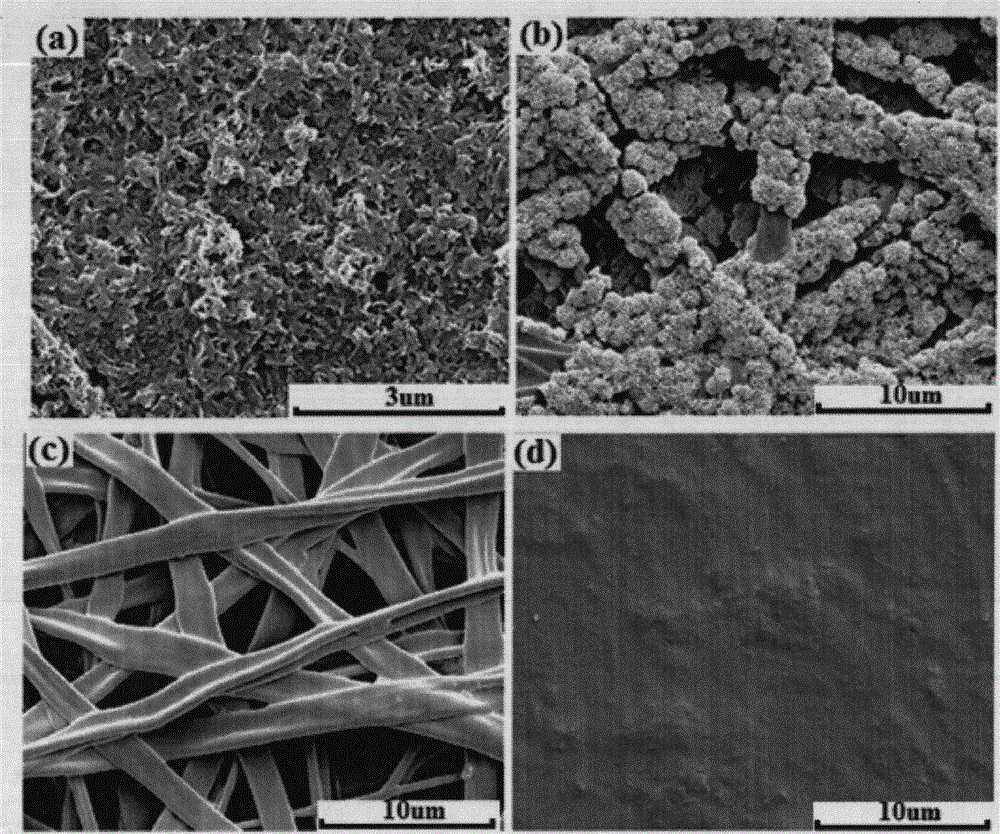
[0023] Example 1: After dissolving 2g of L-PLCA (the molar ratio of LA and CL is 50:50, and the molecular weight is 50,000) in 40ml of acetone, cast it into a film, and dry it at room temperature to obtain a dense film with a thickness of 0.25mm. Then 1.0g L-PLCA (the molar ratio of LA and CL is 70:30, and the molecular weight is 250,000) is added to 10ml of dichloromethane to dissolve, and 0.15g of unmodified n-HA nanoparticles (average particle diameter is 60nm ) with 5ml N, after N dimethylformamide ethanol ultrasonic dispersion, electrospun on above-mentioned casting film and be the film that total thickness is 0.35mm, the fiber of electrospun film is about average 500nm to constitute, and the tensile strength of film is 22MPa, and the elongation at break is 470%. The film was immersed in simulated body fluid for 12 hours, and its surface was completely covered by bone-like apatite as observed by SEM.
Embodiment 2
[0024] Example 2: Add 2g of L-PLCA (the molar ratio of LA to CL is 50:50, and the molecular weight is 300,000) into 50ml of N, N dimethylformamide to dissolve it, cast it into a film, and dry it at room temperature to obtain the thickness It is a dense film of 0.28mm. After adding 1.0g of L-PLCA (the molar ratio of LA to CL is 80:20, and the molecular weight is 200,000) into 6ml of dichloromethane for dissolution, 0.5g of unmodified β-TCP nanoparticles (average particle diameter of 500nm ) after ultrasonic dispersion with 9ml N, N dimethylformamide ethanol, electrospun on the above-mentioned casting film to form a film with a total thickness of 0.37mm, and the electrospun film is composed of fibers with an average thickness of 650nm. The tensile strength of the film is 27MPa, and the elongation at break is 760%. The film is soaked in simulated body fluid for 10 hours, and its surface has been completely covered by bone-like apatite as observed by SEM.
Embodiment 3
[0025]Example 3: 2g of L-PLCA (the molar ratio of LA and CL is 70:30, the molecular weight is 250,000) was dissolved in 35ml of acetone, cast into a film, and dried at room temperature to obtain a dense film with a thickness of 0.26mm. After adding 1.0gL-PLCA (the molar ratio of LA and CL is 70:30, and the molecular weight is 300,000) to 6ml of dichloromethane for dissolution, 0.8g of modified n--HA nanoparticles (average particle diameter is 350nm) After ultrasonically dispersing with 12ml of N, N dimethylformamide ethanol, electrospun on the cast film above to form a film with a total thickness of 0.35mm, the electrospun film is composed of fibers with an average thickness of 830nm. The tensile strength of the film is 30MPa. The elongation at break is 650%. The film is immersed in simulated body fluid for 10 hours, and its surface has been completely covered by bone-like apatite as observed by SEM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com