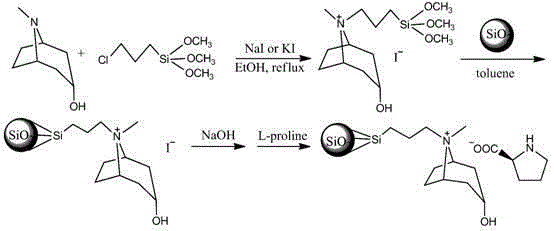
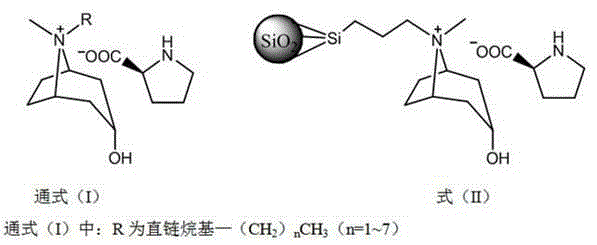
Method for preparing novel tropenol amino acid anionic chiral ionic liquid, immobilization method thereof and method for resolving DL-phenylalanine and DL-tryptophan by using same
A technology of chiral ionic liquid and ionic liquid, which is applied in the field of extraction separation and chromatographic separation, can solve the problems of difficult post-treatment of salt content, and achieve the effects of avoiding the use of organic solvents, reducing the amount of salt, and good environmental protection characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
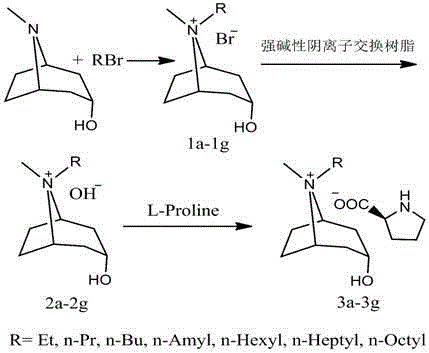
[0027] Embodiment 1 (synthesis of ionic liquid)
[0028] Add 0.05mol tropinol to a 100mL single-necked round-bottom flask, dissolve it with 50mL ethyl acetate, then add 0.06mol bromoethane, heat up to 40°C, reflux and stir for 12h, and filter to obtain a white bromium salt with a yield of 92.60 %. Weigh 0.01 mol of bromium salt, dissolve it with 50 mL of deionized water, and transfer it to a chromatography column equipped with 50 g of strong basic polystyrene anion exchange resin. After staying for 15 minutes, the liquid in the column was slowly released, and the part with pH greater than or equal to 8 was collected. Then slowly drop it into a single-necked flask filled with 0.011mol L-proline, and stir at room temperature for 12h. Remove water under reduced pressure at 60°C, add 100mL of acetonitrile, stir vigorously for 2h, remove insoluble matter by filtration, and remove acetonitrile under reduced pressure at 40°C to obtain light yellow viscous transparent liquid, which ...
Embodiment 2
[0029] Embodiment 2 (synthesis of ionic liquid)
[0030] Add 0.05mol tropinol to a 100mL single-necked round bottom flask, dissolve it with 50mL acetonitrile, then add 0.055mol n-bromobutane, raise the temperature to 80°C, reflux and stir for 16h, and filter to obtain a white bromium salt with a yield of 88.32 %. Weigh 0.01 mol of bromium salt, dissolve it with 50 mL of deionized water, and transfer it to a chromatography column equipped with 50 g of strong basic polystyrene anion exchange resin. After staying for 15 minutes, the liquid in the column was slowly released, and the part with pH greater than or equal to 8 was collected. Then slowly drop it into a single-necked flask filled with 0.012mol L-proline, and stir at room temperature for 16h. Remove water under reduced pressure at 60°C, add 100 mL of acetonitrile, stir vigorously for 2 hours, remove insoluble matter by filtration, remove acetonitrile under reduced pressure at 40°C to obtain light yellow viscous transpar...
Embodiment 3
[0031] Embodiment 3 (synthesis of ionic liquid)
[0032] Add 0.05mol tropinol to a 100mL single-necked round-bottom flask, dissolve it with 50mL acetone, then add 0.05mol n-bromohexane, heat up to 60°C, reflux and stir for 20h, and filter to obtain a white bromium salt with a yield of 79.38% . Weigh 0.01 mol of bromium salt, dissolve it with 50 mL of deionized water, and transfer it to a chromatography column equipped with 50 g of strong basic polystyrene anion exchange resin. After staying for 15 minutes, the liquid in the column was slowly released, and the part with pH greater than or equal to 8 was collected. Then slowly drop it into a single-necked flask filled with 0.012mol L-proline, and stir at room temperature for 20h. Remove water under reduced pressure at 60°C, add 100mL of acetonitrile, stir vigorously for 2h, remove insoluble matter by filtration, and remove acetonitrile under reduced pressure at 40°C to obtain a light yellow viscous transparent liquid, which is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com