Method for preparing sodium stannate from copper anode mud silver separation residue
A technology of copper anode slime and sodium stannate, applied in the direction of improving process efficiency, etc., can solve problems such as the difficulty of recycling tin, and achieve the effects of high recovery rate, simple equipment, and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
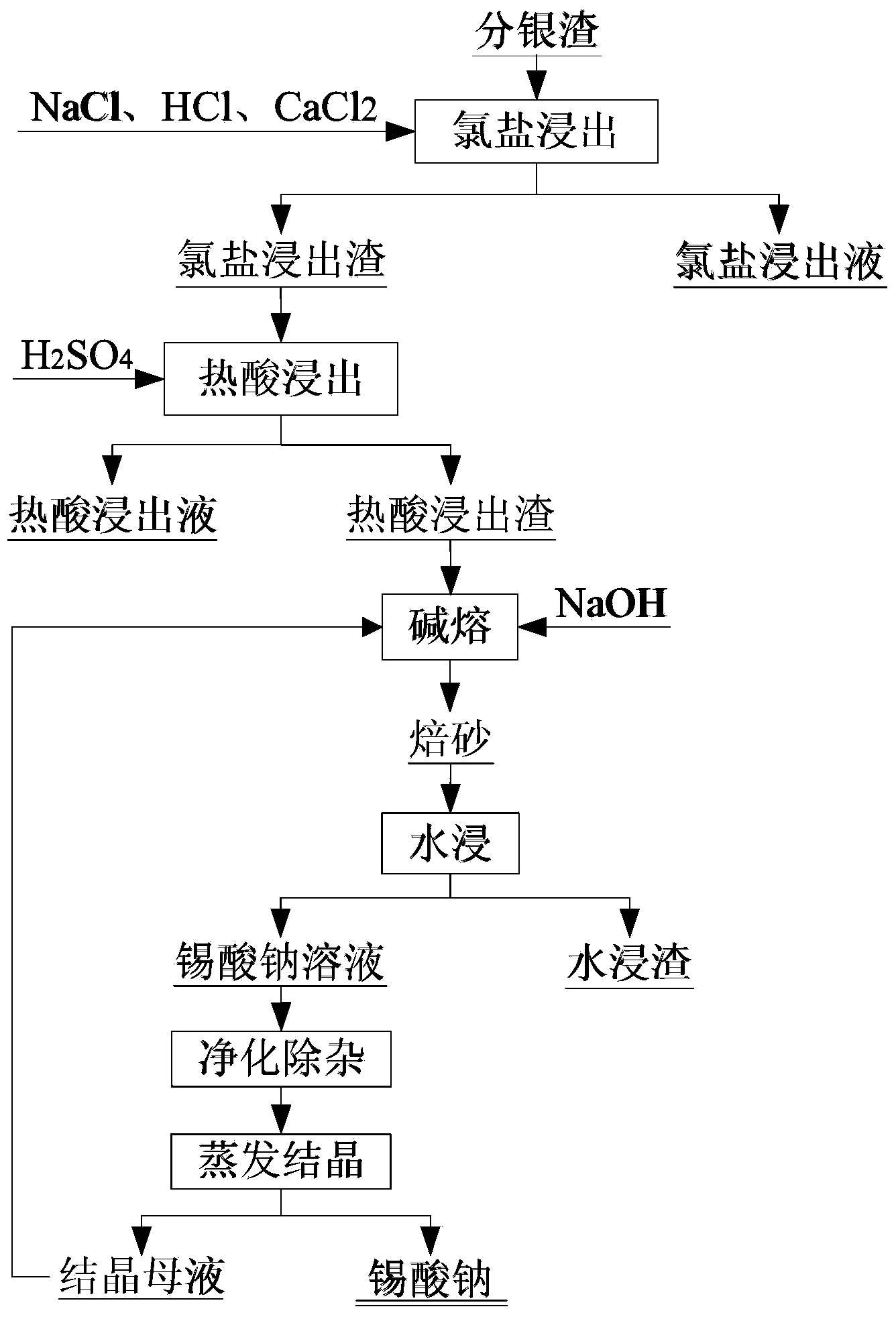
[0025] The main components of the silver separating slag are (%) by weight percentage: Pb15.0, Ag1.76, Ba30.97, Sn7.2.
[0026] Add 480ml of water, 154g of sodium chloride, 53.4ml of 6mol / L hydrochloric acid, and 3.7g of calcium chloride to 50g of silver slag, stir at 80°C for 1 hour, and filter to obtain chlorine salt leaching solution and chloride salt leaching residue; chloride salt leaching residue Add 90ml of concentrated sulfuric acid, leaching at 250°C for 45 minutes to obtain hot acid leaching solution and hot acid leaching slag; add 13g of sodium hydroxide to the hot acid leaching slag, mix evenly and alkali melt at 700°C for 3 hours to obtain calcined sand; After the calcined sand was left to cool to normal temperature, add 80ml of water, stir at 40°C for 1.5 hours, filter to obtain sodium stannate solution and water leaching residue; keep the temperature of sodium stannate solution at 60°C, stir at 300r / min, add The percentage concentration is 5% Na 2 S solution, u...
Embodiment 2
[0028] The main components of the silver separating slag are (%) by weight percentage: Pb12.45, Ag3.86, Ba28.85, Sn8.1.
[0029] Add 900ml of water, 270g of sodium chloride, 95.73ml of 6mol / L hydrochloric acid, and 7.8g of calcium chloride to 100g of silver-separating slag, stir at 90°C for 0.5 hours, and filter to obtain chlorine salt leaching solution and chloride salt leaching residue; chloride salt leaching residue Add 200ml of concentrated sulfuric acid, leaching at 400°C for 35 minutes to obtain hot acid leaching solution and hot acid leaching slag; add 20g of sodium hydroxide to the hot acid leaching slag, mix well, and then perform alkali fusion at 800°C for 1 hour to obtain calcined sand; Let the calcined sand cool to room temperature, add 120ml of water, stir at 30°C for 1 hour, filter to obtain sodium stannate solution and water leaching residue; keep the temperature of sodium stannate solution at 80°C, stir at 400r / min, add mass Min concentration is 10% Na 2 S sol...
Embodiment 3
[0031] The main components of the silver separating slag are (%) by weight percentage: Pb9.78, Ag4.13, Ba24.92, Sn10.3.
[0032] Add 1220ml of water, 415g of sodium chloride, 134ml of 6mol / L hydrochloric acid, and 15g of calcium chloride to 150g of silver slag, stir at 60°C for 2 hours, filter to obtain the chlorine salt leaching solution and chloride salt leaching residue; Sulfuric acid 400ml, leaching at 450°C for 20 minutes to obtain hot acid leaching solution and hot acid leaching slag; adding 30g of sodium hydroxide to the hot acid leaching slag, mixing evenly, and alkali melting at 600°C for 2 hours to obtain calcined sand; Leave to cool to room temperature, add 150ml of water, stir at 50°C for 2 hours, filter to obtain sodium stannate solution and water leaching residue; keep the temperature of sodium stannate solution at 90°C, stirring speed at 300r / min, add mass percent concentration 20% Na 2 S solution, until no black precipitate is produced, after the precipitate i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com