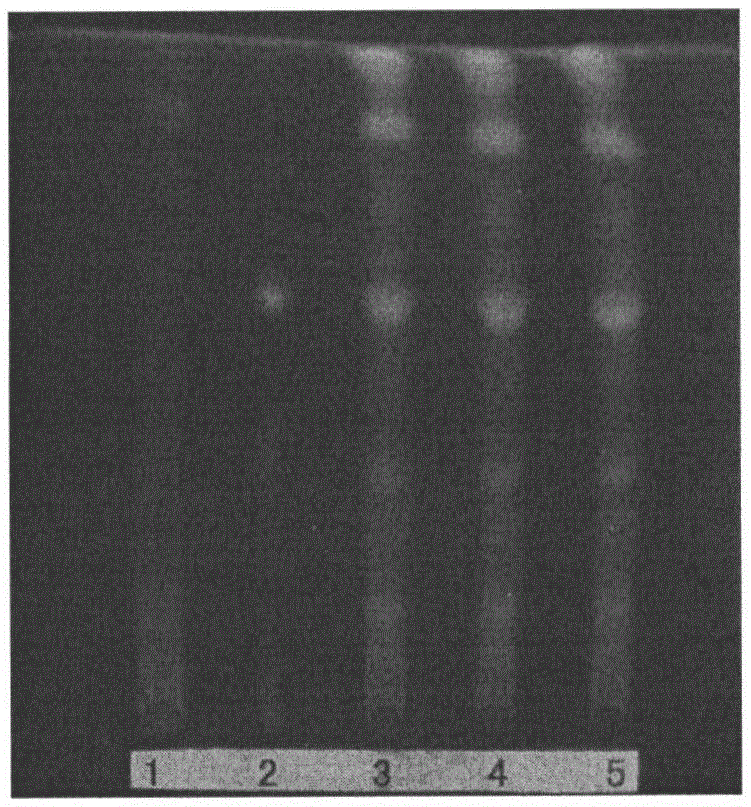
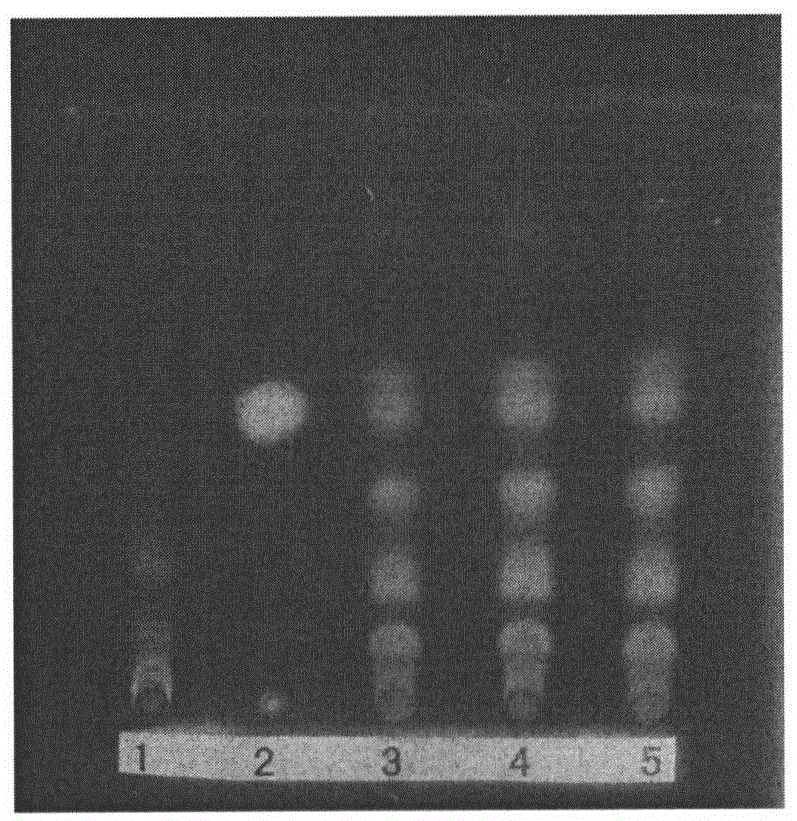
Preparation method and detection method of Gongyanping dispersible tablet
A technique for Gongyanping dispersible tablets and a detection method, which is applied in the field of preparation of Gongyanping dispersible tablets, and can solve the problems of undisclosed components of Gongyanping dispersible tablets, undisclosed Gongyanping dispersible tablets, and undisclosed preparation of Gongyanping dispersible tablets. Evenly dispersible tablet detection method and other problems, to achieve the effect of shortening the disintegration time limit, reducing the quality of control, and stable and uniform quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0048] Diren 450g Liangmianzhen 170g Angelica 140g
[0049] Five Fingers Peach 100g Piercing Stone 140g Microcrystalline Cellulose 110g
[0050] Cross-linked polyvinyl pyrrolidone 35g starch 35g magnesium stearate 0.86g
[0051] Concrete preparation method comprises the following steps:
[0052] (1) Decoction: Take 450g of the above medicinal materials Diren, 170g of Liangmianzhen, 140g of Angelica, 100g of Wuzhi Maotao, 140g of Chuanposhi, add 6 times the amount of water and decoct twice, each time for 2 hours, filter, and combine the decoction , the filtrate is set aside,
[0053] (2) Concentration and alcohol precipitation: Take the above-mentioned filtrate and concentrate it under reduced pressure (-0.04Mpa~0.06Mpa) to form an extract with a relative density of 1.25 (55~60°C); Then add ethanol, and make the alcohol content reach 50%, let it stand for 24 hours,
[0054] (3) Recovery of ethanol and concentration: the above-mentioned alcohol-precipitated drug solution is ...
experiment example 1
[0072] Experimental example 1: Investigation of the amount of water added in the decoction process
[0073] This project adopts the orthogonal design to optimize the process conditions, and investigates the three factors of water addition, decoction time and decoction times respectively, and uses the orthogonal table L 9 (3 4 ) for the test, the schemes are shown in Tables 1 and 2, and the experimental methods and results are shown in Tables 3 and 4.
[0074] Table 1
[0075]
[0076] Table 2 Level arrangement of water decoction process factors
[0077]
[0078] Detection index: Since the medicinal material of Diren is a monarch drug, and Diren contains gallic acid, the detection index takes the extraction rate of gallic acid as the inspection index. According to the quality standards of Diren medicinal materials, the gallic acid contained in Diren shall not be less than 0.05%. The measured gallic acid content was 0.07%.
[0079] Preparation and determination of the ...
experiment example 2
[0093] Experimental Example 2: Optimization of drying method and investigation of paste yield
[0094] The drying method of the thick paste in the present invention usually adopts normal temperature and pressure drying or vacuum drying, for this reason we get 2 prescriptions (2000g) medicinal materials altogether 4 parts, add 6 times of amount water respectively and decoct twice, each time 2 hours, filter After that, combine the filtrates, concentrate to a relative density of 1.25 (55-60°C), add ethanol until the alcohol content reaches 50%, let it stand for 24 hours, filter, recycle the ethanol from the filtrate, concentrate to different relative densities respectively, and use Normal temperature and pressure drying (atmospheric pressure, 60-80°C) or vacuum drying (-0.06-0.08Mpa, 60-80°C), the results are shown in Table 5.
[0095] Table 5 The time spent in different drying methods and the paste yield of samples
[0096]
[0097]
[0098] The results in Table 5 show th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com