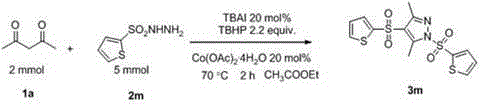
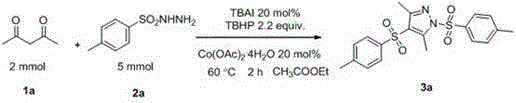
Preparation method of sulfonyl-containing completely substituted parazole
A fully substituted pyrazole technology, applied in the field of preparation of fully substituted pyrazoles, can solve problems such as limited application and complex starting material synthesis operations, and achieve the effects of reduced production costs, wide application range, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] TBAI (20 mol%, 148 mg), compound 1a (2 mmol, 200 mg), compound 2a (5 mmol, 930 mg), Co(OAc) 2 . 4H 2 O (20 mol%, 100 mg), TBHP (0.6 mL, 4.4 mmol), ethyl acetate (8.0 mL). Then the system was heated in the air at 70°C for about 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (40 mL × 3), and then adsorbed with silica gel to dry the solvent in vacuo, finally with petroleum ether and The mixed solvent of dichloromethane can be obtained by simple column chromatography 3a , the yield is 85%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.91 – 7.83 (m, 2H), 7.76 – 7.70 (m, 2H), 7.40 – 7.26 (m, 4H), 2.85 (s, 3H), 2.48 – 2.32 (m, 9H); 13 C NMR (100 MHz, CDCl 3 )δ 151.1, 146.6, 146.5, 144.4, 139.1, 133.8, 130.2, 129.8, 128.0, 126.6, 121.7, 21.6, 21.4, 13.6, 11.6; MS (ESI-quadrupole): Anal. Calcd. For C 19 h 21 N 2 o 4 S 2 : 405, Found: 405 (M+H + ); IR (neat, cm -1 ): υ 1585, 1549, 1171, 660.
Embodiment 2
[0026]
[0027] TBAI (20 mol%, 148 mg), compound 1a (2 mmol, 200 mg), compound 2a (5 mmol, 930 mg), Co(OAc) 2 . 4H 2 O (20 mol%, 100 mg), TBHP (0.6 mL, 4.4 mmol), toluene (8.0 mL). Then the system was heated in the air at 70°C for about 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (40 mL × 3), and then adsorbed with silica gel to dry the solvent in vacuo, finally with petroleum ether and The mixed solvent of dichloromethane can be obtained by simple column chromatography 3a , the yield is 80%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.91 – 7.83 (m, 2H), 7.76 – 7.70 (m, 2H), 7.40 – 7.26 (m, 4H), 2.85 (s, 3H), 2.48 – 2.32 (m, 9H); 13 C NMR (100 MHz, CDCl 3 )δ 151.1, 146.6, 146.5, 144.4, 139.1, 133.8, 130.2, 129.8, 128.0, 126.6, 121.7, 21.6, 21.4, 13.6, 11.6; MS (ESI-quadrupole): Anal. Calcd. For C19 h 21 N 2 o 4 S 2 : 405, Found: 405 (M+H + ); IR (neat, cm -1 ): υ 1585, 1549, 1171, 660.
Embodiment 3
[0029]
[0030] KI (20 mol%, 66 mg), compound 1a (2 mmol, 200 mg), compound 2a (5 mmol, 930 mg), Co(OAc) 2 . 4H 2 O (20 mol%, 100 mg), TBHP (0.6 mL, 4.4 mmol), ethyl acetate (8.0 mL). Then the system was heated in the air at 70°C for about 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (40 mL × 3), and then adsorbed with silica gel to dry the solvent in vacuo, finally with petroleum ether and The mixed solvent of dichloromethane can be obtained by simple column chromatography 3a , the yield is 75%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.91 – 7.83 (m, 2H), 7.76 – 7.70 (m, 2H), 7.40 – 7.26 (m, 4H), 2.85 (s, 3H), 2.48 – 2.32 (m, 9H); 13 C NMR (100 MHz, CDCl 3 )δ 151.1, 146.6, 146.5, 144.4, 139.1, 133.8, 130.2, 129.8, 128.0, 126.6, 121.7, 21.6, 21.4, 13.6, 11.6; MS (ESI-quadrupole): Anal. Calcd. For C 19 h 21 N 2 o 4 S 2 : 405, Found: 405 (M+H + ); IR (neat, cm -1 ): υ 1585, 1549, 1171, 660.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com