Method for preparing organosilicon modified cationic waterborne polyurethane
A water-based polyurethane and organosilicon technology, applied in the field of preparation of organosilicon-modified cationic water-based polyurethane, can solve the problems of acceleration, difficult control of synthesis process, complicated process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
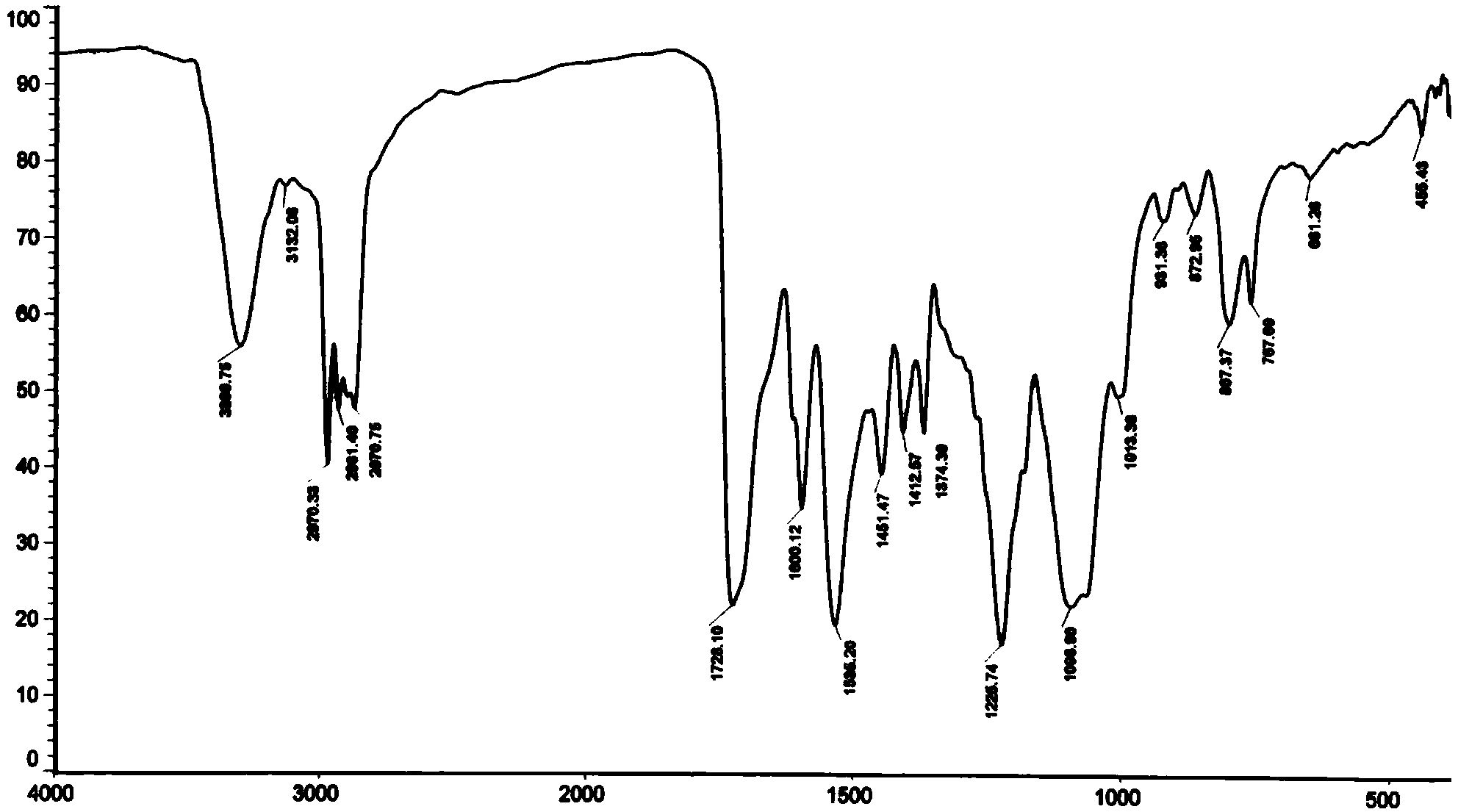
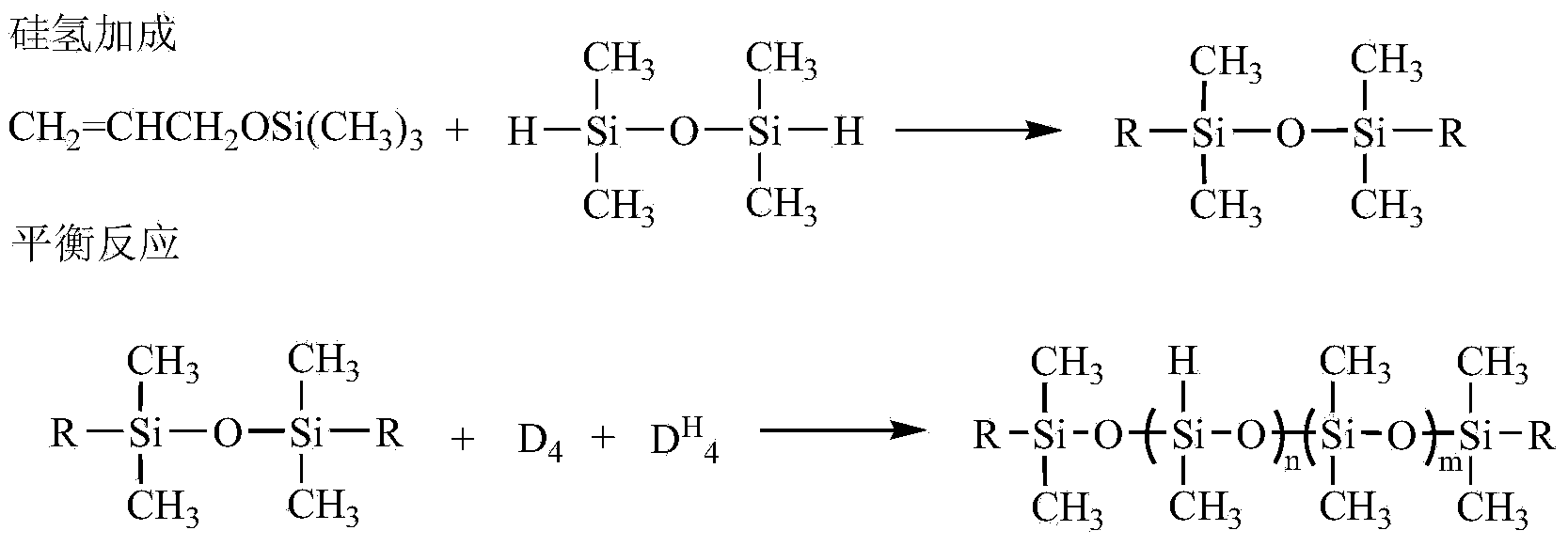
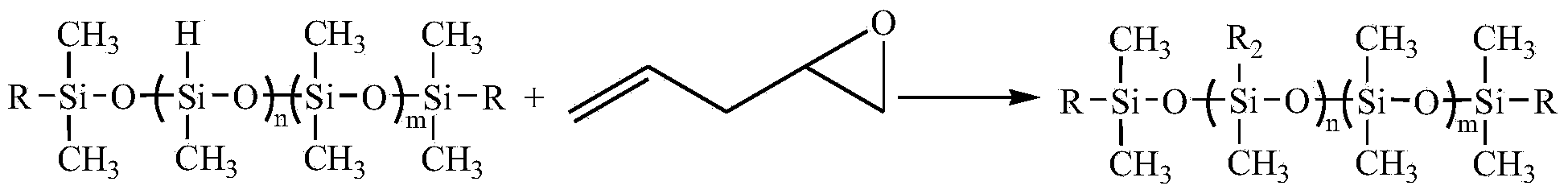
[0062] Silicone with hydroxyl groups at both ends and epoxy groups at the side groups (R 1 =A 1 , R 2 =B 1 , m=5, n=8, M n =2000) synthetic technical route:
[0063] Hydroxyl protection reaction (molar ratio of allyl alcohol: hexamethyldisilazane = 2: 1.1)
[0064] In a 1L three-necked flask, slowly add 355.2g of hexamethyldisilazane to 232.3g of allyl alcohol dropwise at room temperature. After the dropwise addition, raise the temperature of the reaction system to 100°C and continue the reaction at this temperature After 6 hours, the reaction stopped. Atmospheric pressure distillation collected fractions at 98-100°C to obtain 447.5 g of allyloxytrimethylsilane with a yield of 86%.
[0065] Hydrosilylation reaction (molar ratio allyloxytrimethylsilane: tetramethyldisiloxane: chloroplatinic acid = 2.4: 1: 3.1 × 10 -4 )
[0066] In a 1L four-necked bottle, add allyloxytrimethylsilane 312.5g, 50mL toluene and chloroplatinic acid 3.1×10 -4 mol of isopropanol solution, aft...
Embodiment 2
[0073] Embodiment 2 A kind of preparation method of organosilicon modified cationic waterborne polyurethane
[0074] Prepolymerization
[0075] In a reaction flask equipped with a stirrer and a thermometer, add 174g of toluene diisocyanate and 100g of organosilicon (R 1 =A 1 , R 2 =B 1 , m=5, n=8, M n =2000), heated up to 70°C and reacted for 0.5h; then added 200g polyether diol (M n =800), the reaction was continued for 0.5h at the same temperature.
[0076] Chain extension and capping reactions
[0077] Add 12.4g of ethylene glycol into the reaction system, react at 70°C for 0.5h, and use the standard di-n-butylamine back titration method to analyze the isocyanate group content in the prepolymer. When the isocyanate group content reaches the theoretical value of 8.63 %, add 74g monohydroxy glycidyl ether (R 5 = H, M n =74) Terminate the reaction to obtain a silicone-modified polyurethane whose main chain contains ether bonds, urethane bonds and polysiloxane segments...
Embodiment 3
[0083] Embodiment 3 A kind of preparation method of organosilicon modified cationic waterborne polyurethane
[0084] Prepolymerization
[0085] In a reaction flask equipped with a stirrer and a thermometer, add 222g of isophorone diisocyanate and 150g of organosilicon (R 1 =A 1 , R 2 =B 8 , m=9, n=2, M n =1500), heated up to 70°C and reacted for 0.5h; then added 250g of polyether glycol (M n =1000), the reaction was continued for 0.5h at the same temperature.
[0086] Chain extension and capping reactions
[0087] Add 6.2g of ethylene glycol into the reaction system, react at 70°C for 0.5h, and then use the standard di-n-butylamine back titration method to analyze the isocyanate group content in the prepolymer. When the isocyanate group content reaches the theoretical value of 7.35 %, add 114.4g monohydroxy glycidyl ether (R 5 =OHCH 2 ,M n =104) terminate the reaction to obtain a silicone-modified polyurethane containing ether bonds, urethane bonds and polysiloxane s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com